Abstract
Objectives
The subcommittee on scrotal imaging, appointed by the board of the European Society of Urogenital Radiology (ESUR), have produced guidelines on imaging and follow-up in testicular microlithiasis (TML).
Methods
The authors and a superintendent university librarian independently performed a computer-assisted literature search of medical databases: MEDLINE and EMBASE. A further parallel literature search was made for the genetic conditions Klinefelter’s syndrome and McCune-Albright syndrome.
Results
Proposed guidelines are: follow-up is not advised in patients with isolated TML in the absence of risk factors (see Key Points below); annual ultrasound (US) is advised for patients with risk factors, up to the age of 55; if TML is found with a testicular mass, urgent referral to a specialist centre is advised.
Conclusion
Consensus opinion of the scrotal subcommittee of the ESUR is that the presence of TML alone in the absence of other risk factors is not an indication for regular scrotal US, further US screening or biopsy. US is recommended in the follow-up of patients at risk, where risk factors other than microlithiasis are present. Risk factors are discussed and the literature and recommended guidelines are presented in this article.
Key Points
• Follow up advised only in patients with TML and additional risk factors.
• Annual US advised for patients with risk factors up to age 55.
• If TML is found with testicular mass, urgent specialist referral advised.
• Risk factors – personal/ family history of GCT, maldescent, orchidopexy, testicular atrophy.
Similar content being viewed by others
Introduction
Testicular microlithiasis (TML) is a common finding on scrotal ultrasound (US), but its significance remains controversial. In September 2012, the board of the European Society of Urogenital Radiology (ESUR) established a subcommittee on scrotal imaging which was asked to produce guidelines on imaging and follow-up in TML.
Process
The guidelines were written by consensus, based on expert opinion from members of the Scrotal Imaging Working Group of the ESUR, and following review of the literature. This consensus was reached after three face-to-face meetings of the subcommittee, communication amongst the subcommittee members by email and sharing of research findings and papers. Preliminary findings were discussed at committee meetings and all members agreed on the guidelines proposed below.
The authors and a superintendent university librarian independently performed a computer-assisted literature search of medical databases: MEDLINE (January 1951 to date) and EMBASE (January 1974 to date). The search parameters are summarised in Table 1. A further, parallel literature search was made for the genetic conditions Klinefelter’s syndrome and McCune-Albright syndrome, summarised in Table 2. All the references were imported into Endnote Professional Edition Version 7 © 1988–2013 Thompson Reuters and each study reviewed.
Results
The presence of TML alone in the absence of other risk factors is not an indication for regular scrotal US, further US screening or biopsy. US may be recommended in the follow-up of patients at risk, when risk factors other than microlithiasis are present.
Testicular microlithiasis (TML)
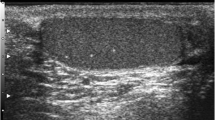
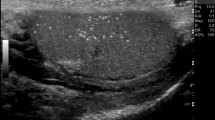
TML is a condition in which calcium deposits form in the lumina of seminiferous tubules or arise from the tubular basement membrane components [1–4]. The histology shows micro calcium deposits with surrounding fibrosis. The microliths do not cause pain or symptoms and are impalpable. TML is an imaging diagnosis, almost invariably made with testicular US, where an echogenic non-shadowing focus less than 3 mm will be seen.
Two possible definitions for TML have been proposed: five or more microliths in the whole testis [5, 6], or five or more microliths per field of view. The former is more straightforward— if there are five foci in imaging the testis, there is TML. The latter definition captures the idea of clustering better, but not rigorously. Clustering may be important, and in fact, five microliths per field in a cluster may be more worrying than 10 scattered throughout the testis. The clustering may herald a dysgenic ‘unstable’ area in the testis, wherein carcinoma in situ (CIS) can develop. In any case, a cluster of five microliths would fulfil the first definition; the committee declared an overwhelming preference for the second definition. The microcalcifications are not visible on MRI.
Testicular ultrasound
The testes are ideally suited to US evaluation due to their superficial position within the scrotum, which permits imaging with high frequency linear array transducers, producing images of high resolution. A number of advances in US technology in recent years have further increased US image quality; it is likely that this has resulted in increased detection of testicular microliths. The major advance in image quality has been through improved transducer technology. Transducer frequency has been increased such that modern small-part linear array transducers will typically have a centre frequency of at least 12 MHz producing images of high spatial resolution. Development of high bandwidth transducers has also permitted adoption of harmonic imaging technology where the fundamental (transmitted) frequency is removed from the received signal and the image formed from harmonic frequencies; this contributes to higher image quality by reduction of artefacts due to clutter from beam side lobes and reverberation artefacts. A number of innovations in post processing have been developed to increase contrast resolution and reduce speckle. These include spatial compounding and frequency compounding. The increase in computing power has underpinned these developments, enabling large volumes of data to be quickly processed. This has allowed the development of a wide variety of post processing algorithms to further improve image quality.
When evaluating the testes, US should be performed with a high frequency transducer of at least 15 MHz. A lower frequency transducer may be considered for large scrotums where US beam penetration may be an issue. We concur with the European Association of Urology (EAU) statement ‘The incidence (of testicular microlithiasis) reported seems to be higher with high-frequency ultrasound machines’ [http://www.ncbi.nlm.nih.gov/pubmed/10524881].
TML may be detected in different clinical scenarios which may require a tailored approach for follow-up. When TML is discovered, it may be useful to complete a checklist for risk factors at the end of the US examination to decide which recommendation to follow. A sample checklist is provided as Appendix 2.
Some common clinical scenarios where TML may be discovered include:
-
1.
As an isolated finding at scrotal US, in the absence of any risk factors (Table 3).
Table 3 Risk factors that warrant follow-up of men with TML The current recommendations, including those of the EAU [7–10] are that the presence of microlithiasis alone is not an indication for a regular scrotal US [9], and that in the absence of other risk factors, TML is not an indication for biopsy or further US screening [8, 10].
The patient may be discharged with advice about performing monthly scrotal self-examination and a patient information leaflet (Appendix 1). The rationale for this approach is discussed further below.
-
2.
When TML is discovered in the setting of another risk factor, regardless of whether it is unilateral or bilateral (Table 3), and provided that there is no focal lesion within either testis, the following may be advised:
-
a.
Annual follow-up with US.
-
b.
Monthly self-examination: the patient may need to be taught the procedure in the urology clinic.
-
c.
If self-examination reveals a new mass within the scrotum, there should be direct access to fast track US, without the need for repeat clinical referral.
An important advantage of the annual surveillance is to maintain the patient’s engagement with the process, as indefinite self-examination without intermittent contact with medical care is likely to fail.
-
a.
-
3.
If TML is discovered together with a focal testicular mass/marked hypoechoic area, immediate referral should be made to a specialist urology centre.
-
4.
If there are too many microliths to adequately assess the testicular parenchyma, or if TML appears clearly asymmetric with alterations of echotexture, guidelines suggest that referral is made to a specialist centre.
Follow-up is recommended up to the age of 55 years, based on European data on the incidence of testicular cancer by age in male populations from UK, France, Greece and Poland. However, doubt exists whether the incidental detection of a subclinical mass on annual screening US confers any survival advantage over early clinical detection achieved by regular self-examination [11].
On referral to a specialist centre, depending on local practice, further tests may include:
-
a.
Measurement of tumour markers
-
b.
Further imaging, such as gadolinium-enhanced MRI or contrast enhanced US
-
c.
Surveillance US
-
d.
Surgical biopsy or orchidectomy
Testicular macrocalcification, that is any intratesticular focus of coarse calcification (larger than TML), separate from any intratesticular mass, may also be found during a testicular US. It has been suggested that patients with any intratesticular calcification should be considered to be at higher risk of a co-existing testicular malignant lesion, and possibly also of developing a neoplasm in the future [12]. Recommendations for surveillance of testicular macrocalcification lie outside the remit of this paper.
MRI
MRI has no direct role in the monitoring of TML and microliths are not visible on MRI. If an intratesticular mass is suspected and US findings are indeterminate, MRI may be used as a problem-solving modality.
Evidence: risk factors
A helpful summary of the risk factors has been presented by Manecksha and Fitzpatrick [13].
Testicular dysgenesis syndrome (TDS)
At the turn of the millennium, around the same time that TML was being proposed as a premalignant condition, papers were published advocating the concept of testicular dysgenesis syndrome.
‘There is evidence that poor semen quality, testicular cancer, undescended testes and hypospadias are symptoms of one underlying entity, testicular dysgenesis syndrome (TDS), which may be increasingly common due to adverse environmental influences.’ The same authors asserted that there was increasing evidence of the importance of TDS in initiating testicular germ cell tumour (GCT) and that it was also associated with microlithiasis [14–16]. Further evidence for the TDS hypothesis came from a UK-based meta-analysis [17] as well as a 2009 review [18] that concluded that ‘Dysgenetic testes often have an irregular ultrasound pattern, where microliths may also be visible. However, the cause of TDS in humans remains to be determined.’
More recently, and mirroring the move away from insisting that TML is premalignant, reports challenge the TDS model. Epidemiological studies provide little support for existence of a widespread TDS because there are no consistent non-causal associations between its different manifestations. There is furthermore little evidence of shared causes between the alleged components of the syndrome [19–21]. A Norwegian study gauging relative fertility between men with GCT and matched control concluded that fatherhood was slightly more frequent among men developing testicular cancer (TC) than in controls. Prediagnosis fertility rates of men who developed TC were similar to those of age-matched men from the general population. Interestingly, men developing TC in both testicles did not have inferior fatherhood rates before diagnosis. These results challenge the appropriateness of the TDS which would predict that infertility would be higher in those predisposed by virtue of TDS to GCT [1].
History of germ cell tumour
Patients with a history of prior malignancy may fall into the following categories:
Family history, 1st degree male relative
Approximately 1.4 % of newly diagnosed GCT patients report a positive family history. The relative risk to a brother of a GCT case is 8–10 and the relative risk to a father/son of a GCT case is 4–6. Moreover, a 37/67.5-fold elevated risk of GCT in dizygotic/monozygotic twin brothers of men with GCT has been reported [22]. In the nationwide Swedish Family-Cancer Database study to analyse the risk for testicular cancer by Hemmink that included 4,856 patients with testicular cancer, aged 0–70 years, standardised incidence ratios for familial risk were 3.8-fold when a father and 7.6-fold when a brother had testicular cancer [23].
Age at diagnosis is 2–3 years younger for familial versus sporadic cases. Mai et al. reported that familial cases on average were diagnosed 2–3 years younger than population cases, with seminoma demonstrating a larger difference [24].
TML is significantly more common among family members of men with GCT than in the general population [22, 25, 26].
Maldescent and/or orchidopexy
These are established as independent risk factors for developing GCTs even in the absence of TML.
Reduced volume of testis
Testicular atrophy should not just be based on testicular volume measurement as testicular morphology is also important in recognition of this condition in ‘acquired’ atrophy rather than in ‘primary hypoplasia’. For patients up to 18 years of age, normative values have been provided by Goede et al. [27]. Beside evaluation of the volume, a difference greater than 20 % between the volume of the two testes should also be considered, to assess atrophy. It is worth noting that the left testis is usually smaller than the right testis.
The size of testes in many publications is evaluated by comparison with some models (Prader orchidometer)—the method overestimates the testicular volume by about 20–25 %, even if it correlates very strongly with the US volumetry. Measurements in US are used to measure the volume, but there are also several formulae. For this document, the normal mean testicular volume is estimated at 18 ml (12–30 ml) with the interpretation that a testis less than 12 ml in volume should be considered as small. Accepting that it may not be routine practice to measure the testicular volume in each case, volumetry should be performed if the maximum testicular dimension is 35 mm or less.
Referred for scrotal ultrasound with relevant genetic disorder
Klinefelter’s syndrome
Sporadic case reports of TML in Klinefelter’s syndrome have been published, although there is no evidence that the incidence is higher than background. The majority of patients with this syndrome have infertility and/or testicular atrophy [28], and TML in association with atrophy should trigger surveillance. In the setting of infertility and TML but otherwise normal scrotal US findings, management should be as for the general population. Incidental testicular nodules (small nodules) are seen more frequently in patients with Klinefelter’s syndrome than in the general population, and most nodules represent benign Leydig cell nodules/hyperplasia.
Testicular pathology in McCune-Albright syndrome
The incidence of gonadal pathology in McCune-Albright syndrome is equal in men and women. Testicular abnormality is seen on US in about 80 % including 30 % with microlithiasis and 11 % focal calcifications [29, 30]. The predominant histopathological finding is Leydig cell hyperplasia, which carries a low risk of malignant transformation and can be managed conservatively. Recommendations for this condition, and for disparate other syndromes including congenital adrenal hyperplasia, are the same as for the general population.
Referred for scrotal ultrasound with infertility
The additional risk, if any, of GCT in men with subfertility, who on scrotal US have TML, is hard to determine, not least because of lack of subclassification within the literature [31]. The group that may warrant follow-up are those with primary infertility, non-obstructive and non-endocrine i.e. testicular aetiology. In this group, the relationship between TML and infertility is unclear, but may relate to dysgenesis of the testes, with degenerate cells being sloughed inside an obstructed seminiferous tubule and failure of the Sertoli cells to phagocytose the debris. Subsequently, calcification occurs [18, 32].
The majority of focal lesions discovered during US for infertility are benign [33].
Paediatric population [33–37]
Scrotal US may be performed in children/adolescents for atrophy, maldescent, in those with a syndrome or in patients with clinical symptoms [34–38]. Recognition of TML in these groups should be managed as follows:
-
1.
Atrophy [27]: annual US follow-up, inform parents/guardians and teach scrotal examination
-
2.
Maldescent/post orchidopexy: annual US follow-up, inform parents/guardians and teach scrotal examination
-
3.
Syndrome: see section above
-
4.
Symptoms: as for general recommendations
Testicular biopsy in TML: recommendations
Patients with small or atrophic testes with microcalcifications/microliths or an irregular echo pattern on US are at increased risk of harbouring CIS [39]. At orchidectomy in men with GCT, if there is TML in the contralateral testis, or if the contralateral testis is atrophic, biopsy of contralateral testis may be indicated to look for CIS. A policy of ‘open access’ wherein the man may refer himself directly for follow-up scrotal US if an intratesticular mass is felt on self-examaintion can be very worthwhile; in our experience it has sped up treatment in men with interval GCTs and has not led to a flood of self-referrals.
Conclusion
The presence of TML alone in the absence of other risk factors is not an indication for regular scrotal US, further US screening or biopsy.
Ultrasound is recommended in the follow-up of patients at risk, where risk factors other than microlithiasis are present. A summary of the guidelines is presented in Table 4.
References
De Jong BW, De Gouveia Brazao CA, Stoop H, Wolffenbuttel KP, Oosterhuis JW, Puppels GJ et al (2004) Raman spectroscopic analysis identifies testicular microlithiasis as intratubular hydroxyapatite. J Urol 171(1):92–96
Kim B, Winter TC, Ryu JA (2003) Testicular microlithiasis: clinical significance and review of the literature. Eur Radiol 13(12):2567–2576
Renshaw AA (1998) Testicular calcifications: incidence, histology and proposed pathological criteria for testicular microlithiasis. J Urol 160(5):1625–1628
Drut R, Monica R (2002) Testicular microlithiasis: histologic and immunohistochemical findings in 11 pediatric cases. Pediatr Dev Pathol 5(6):544–550
Bennett HF, Middleton WD, Bullock AO, Teefey SA (2001) Testicular microlithiasis: US follow-up. Radiology 218(2):359–363
Backus ML, Mack LA, Middleton WD, King BF, Winter TC 3rd, True LD (1994) Testicular microlithiasis: imaging appearances and pathologic correlation. Radiology 192(3):781–785
Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Tournaye H, Krausz C (2013) Guidelines on male infertility: European Association of Urology. Available from: http://www.uroweb.org/gls/pdf/16_Male_Infertility_LR.pdf
Elzinga-Tinke JE, Sirre ME, Looijenga LH, van Casteren N, Wildhagen MF, Dohle GR (2010) The predictive value of testicular ultrasound abnormalities for carcinoma in situ of the testis in men at risk for testicular cancer. Int J Androl 33(4):597–603
DeCastro BJ, Peterson AC, Costabile RA (2008) A 5-year followup study of asymptomatic men with testicular microlithiasis. J Urol 179(4):1420–1403
Montgomery JS, Bloom DA (2011) The diagnosis and management of scrotal masses. Med Clin North Am 95(1):235–244
Tan IB, Ang KK, Ching BC, Mohan C, Toh CK, Tan MH (2010) Testicular microlithiasis predicts concurrent testicular germ cell tumors and intratubular germ cell neoplasia of unclassified type in adults: a meta-analysis and systematic review. Cancer 116(19):4520–4532
Miller FN, Rosairo S, Clarke JL, Sriprasad S, Muir GH, Sidhu PS (2007) Testicular calcification and microlithiasis: association with primary intra-testicular malignancy in 3,477 patients. Eur Radiol 17(2):363–369
Manecksha RP, Fitzpatrick JM (2009) Epidemiology of testicular cancer. BJU Int 104(9 Pt B):1329–1333
Skakkebaek NE (2003) Testicular dysgenesis syndrome. Horm Res 60(Suppl 3):49
Skakkebaek NE, Holm M, Hoei-Hansen C, Jorgensen N, Rajpert-De E, Mayer F et al (2003) Association between testicular dysgenesis syndrome (TDS) and testicular neoplasia: evidence from 20 adult patients with signs of maldevelopment of the testis. APMIS 111(1):1–11
Joensen UN, Jorgensen N, Rajpert-De E, Skakkebaek NE (2008) Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol 102(2):155–161
Martin OV, Shialis T, Lester JN, Scrimshaw MD, Boobis AR, Voulvoulis N (2008) Testicular dysgenesis syndrome and the estrogen hypothesis: a quantitative meta-analysis. Environ Health Perspect 116(2):149–157
Wohlfahrt-Veje C, Main KM, Skakkebaek NE (2009) Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol 71(4):459–465
James WH (2010) Further grounds for abandoning the concept of testicular dysgenesis syndrome: a response to the paper of Akre and Richiardi (2009). Hum Reprod 25(4):1084–1086
Akre O, Richiardi L (2009) Does a testicular dysgenesis syndrome exist? Hum Reprod 24(9):2053–2060
Cvancarova M, Oldenburg J, Sprauten M, Stensheim H, Fossa SD (2011) Reproduction rates prior to diagnosis of testicular cancer: does the testicular dysgenesis syndrome exist? J Clin Oncol 29(15 Suppl 1)
Greene MH, Kratz CP, Mai PL, Mueller C, Peters JA, Bratslavsky G et al (2010) Familial testicular germ cell tumors in adults: 2010 summary of genetic risk factors and clinical phenotype. Endocr Relat Cancer 17(2):R109–R121
Hemminki K, Chen B (2006) Familial risks in testicular cancer as aetiological clues. Int J Androl 29(1):205–210
Mai PL, Chen BE, Tucker K, Friedlander M, Phillips KA, Hogg D et al (2009) Younger age-at-diagnosis for familial malignant testicular germ cell tumor. Fam Cancer 8(4):451–456
Coffey J, Huddart RA, Elliott F, Sohaib SA, Parker E, Dudakia D et al (2007) Testicular microlithiasis as a familial risk factor for testicular germ cell tumour. Br J Cancer 97(12):1701–1706
Korde LA, Premkumar A, Mueller C, Rosenberg P, Soho C, Bratslavsky G et al (2008) Increased prevalence of testicular microlithiasis in men with familial testicular cancer and their relatives. Br J Cancer 99(10):1748–1753
Goede J, Hack WW, Sijstermans K, van der Voort-Doedens LM, Van der Ploeg T, Meij-de Vries A et al (2011) Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr 76(1):56–64
Okada H, Fujioka H, Tatsumi N, Kanzaki M, Okuda Y, Fujisawa M et al (1999) Klinefelter’s syndrome in the male infertility clinic. Hum Reprod 14(4):946–952
Boyce AM, Chong WH, Shawker TH, Pinto PA, Linehan WM, Bhattacharryya N et al (2012) Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab 97(9):E1782–E1790
Daneman A, Daneman D (2007) McCune-Albright syndrome. J Pediatr Endocrinol Metab 20(12):1265
Thomas K, Wood SJ, Thompson AJ, Pilling D, Lewis-Jones DI (2000) The incidence and significance of testicular microlithiasis in a subfertile population. Br J Radiol 73(869):494–497
Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K et al (2011) EAU guidelines on testicular cancer: 2011 update. Eur Urol 60(2):304–319
Butruille C, Marcelli F, Ghoneim T, Lemaitre L, Puech P, Leroy X et al (2012) Management of testicular lesions in a population of infertile patients. [French] Prise en charge des nodules testiculaires dans une population de patients infertiles. Prog Urol 22(1):45–52
Furness PD 3rd, Husmann DA, Brock JW 3rd, Steinhardt GF, Bukowski TP, Freedman AL et al (1998) Multi-institutional study of testicular microlithiasis in childhood: a benign or premalignant condition? J Urol 160(3 Pt 2):1151–1154, discussion 78
Goede J, Hack WWM, van der Voort-Doedens LM, Sijstermans K, Pierik FH (2009) Prevalence of testicular microlithiasis in asymptomatic males 0 to 19 years old. J Urol 182(Suppl 4):1516–1520
Chiang LW, Yap TL, Asiri MM, Phaik Ong CC, Low Y, Jacobsen AS (2012) Implications of incidental finding of testicular microlithiasis in paediatric patients. J Pediatr Urol 8(2):162–165
Goede J, Hack WW (2012) Clinical aspects of testicular microlithiasis in boys: a review. J Pediatr Urol 8(5):459–469
Deganello A, Svasti-Salee D, Allen P, Clarke JL, Sellars ME, Sidhu PS (2012) Scrotal calcification in a symptomatic paediatric population: prevalence, location, and appearance in a cohort of 516 patients. Clin Radiol 67(9):862–867
Hoei-Hansen CE, Olesen IA, Jorgensen N, Carlsen E, Holm M, Almstrup K et al (2007) Current approaches for detection of carcinoma in situ testis. Int J Androl 30(4):398–404, discussion 404–405
Acknowledgments
The scientific guarantor of this publication is Jonathan Richenberg. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was not required because not applicable. Methodology: prospective/retrospective
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Patient leaflet for testicular microlithiasis
What is testicular microlithiasis?
Small lumps of calcium lie in the small tubes within the testicle. There must be at least 5 such calcifications in one (or both) testicles before the label testicular microlithiasis (TML) is applied. TML is seen in about 2 or 3 men in every hundred.
How is TML detected?
The calcium lumps cannot be felt and they do NOT cause discomfort. They can only be seen on ultrasound. In other words, TML was discovered incidentally during your ultrasound scan of the testes.
Why is TML important?
At the end of the 1990s, there was some concern that TML might lead to cancer of the testicle. Since then, many studies across the world have looked at TML. They have NOT confirmed the initial worries.
There is no evidence that TML on its own leads to cancer.
What should I do?
Like every man, including men who do not have TML, you should practice monthly self-examination of the testicles. If you are uncertain about how to do this, please ask your doctor. Nothing else is required. You do not need regular ultrasound scans. The calcium in the testicles is not related to your diet or to any sexual or other activity.
What should I do if I feel a new lump during self-examination?
Please contact your family doctor or specialist. Your family doctor or specialist will examine you and if thought appropriate will refer you on for a specialist opinion. It is likely that you will be referred also for an urgent ultrasound scan. This will be usually at the hospital where the initial scan was performed.
Appendix 2
Checklist to be completed in men discovered to have TML
If you discover a patient has TML during ultrasound scanning, risk factors for developing GCT should be ascertained.
Risk factor | Comments | Yes >=5 ML per FoV | Yes Diffuse | No TML i.e. no FoV contains 5 or more microliths |
|---|---|---|---|---|
Maldescent | Ask patient for relevant history | Annual US | Annual US | Discharge |
Orchidopexy | Ask patient for relevant history | Annual US | Annual US | Discharge |
Previous GCT | Likely to have orchidectomy so this should be easy to ascertain. If there is any doubt, ask the patient | Annual US | Annual US | Discharge |
Genetic disease | Ask patient for relevant history | Repeat US at 6 and 12 months, D/C if no nodule >3mm | Refer | Discharge |
Family history of GCT | Ask patient for relevant history | Encourage self-examination and offer open access | Encourage self-examination and offer open access | Discharge |
Atrophic testis | Should be noted during the ultrasound examination | Annual US | Annual US | Discharge |
Rights and permissions
About this article
Cite this article
Richenberg, J., Belfield, J., Ramchandani, P. et al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol 25, 323–330 (2015). https://doi.org/10.1007/s00330-014-3437-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3437-x