Abstract
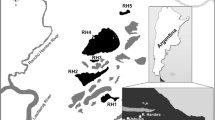
Microbial communities living in microbial mats are known to constitute early indicators of ecosystem disturbance, but little is known about their response to environmental factors in the Antarctic. This paper presents the first major study on ciliates from microbial mats in streams on King George Island (Antarctica). The objective of the study is to investigate the structure and spatial distribution of ciliate communities and to identify the environmental factors determining the structure of the assemblages. Additionally, we compared ciliate communities inhabiting streams on King George Island with other isolated islands of the maritime Antarctic. Samples of microbial mats for ciliate analysis were collected from three streams fed by Ecology Glacier. Sampling was carried out three times from 17 January to 24 February 2012. The total numbers of ciliates were dominated by small taxa recognized as cosmopolitan, also recorded on other islands of the maritime Antarctic. The species richness, abundance, and biomass of ciliates differed significantly between the stations studied, with the lowest numbers in the middle course of the stream and the highest numbers in the microhabitats closest to the glacier and at the site where the stream empties into the pond. Variables that significantly explained the variance in ciliate communities in the transects investigated were total organic carbon, total nitrogen, temperature, dissolved oxygen, and conductivity. Further research is required to explain the impact of biotic factors on the presence of ciliates, including the abundance of bacteria, microalgae, and small metazoa.





Similar content being viewed by others
References
Alcántara-Hernández RJ, Centeno CM, Mendoza-Ponce A, Batista S, Ibarra-Merino M, Campo J, Falcón lf (2014) Characterization and comparison of potential denitrifiers in microbial mats from King George Island, Maritime Antarctica. Polar Biol 37:403–416. doi:10.1007/s00300-01301440-3
Augustin H, Foissner W, Adam H (1984) An improved pyridinated silver carbonate method which needs few specimens and yields permanent slides of impregnation ciliates (Protozoa, Ciliophora). Mikroskopie 41:134–137
Bamforth SS, Wall DH, Virginia RA (2005) Distribution and diversity of soil protozoa in the McMurdo Dry Valleys of Antarctica. Polar Biol 28:756–762. doi:10.1007/s00300-005-0006-4
Beyens L, Chardez D, de Baere D, Verbruggen C (1995) The aquatic testate amoebae fauna of the Stomness Bay area, South Georgia. Antarctic Sci 1:3–8. doi:10.1017/S0954102095000022
Bintanja R (1995) The local surface energy balance of the Ecology Glacier, King George Island, Antarctica: measurements and modeling. Antarctic Sci 3:315–325. doi:10.1017/S0954102095000435
Bonilla S, Villeneuve V, Vincent WF (2005) Benthic and planktonic algal communities in a high arctic lake: pigment structure and contrasting responses to nutrient enrichment. J Phycol 41(6):1120–1130. doi:10.1111/j.1529-8817.2005.00154.x
Camacho A (2006) Planktonic microbial assemblages and the potential effects of metazooplankton predation on the food web of lakes from the maritime Antarctica and sub-Antarctic islands. Rev Environ Sci Biotechnol 5:167–185. doi:10.1007/s11157-006-0003-2
Chong CW, Pearce DA, Convey P, Tan GYA, Wong RCS, Tan IKP (2010) High levels of spatial heterogeneity in the biodiversity of soil prokaryotes on Signy Island, Antarctica. Soil Biol Biochem 42:601–610. doi:10.1016/j.soilbio.2009.12.009
Chróst RJ, Siuda W (2006) Microbial production, utilization, and enzymatic degradation of organic matter in the upper trophogenic layer in the pelagial zone of lakes along a eutrophication gradient. Limnol Oceanogr 5:749–762
De los Rios A, Ascaso C, Wierzchos J, Fernández-Valiente E, Quesada A (2004) Microstructural characterization of cyanobacterial mats from the McMurdo Ice Shelf, Antarctica. Appl Environ Microbiol 70:569–580. doi:10.1128/aem.70.1.569-580.2004
De Wever A, Leliaert F, Verleyen E, Vanormelingen P, Van der Gucht K, Hodgson DA, Sabbe K, Vyverman W (2009) Hidden levels of phylodiversity in Antarctic green algae: further evidence for the existence of glacial refugia. Proc R Soc B Biol Sci 276:3591–3599. doi:10.1098/rspb.2009.0994
Di Castri F, Hansen AJ, Holland MM (1988) A new look at ecotones: emerging international projects on landscape boundaries. Biol Int 17:1–163
Dietrich D, Arndt H (2004) Benthic heterotrophic flagellates in an Antarctic melt water stream. Hydrobiologia 529:59–70. doi:10.1007/s10750-004-4947-3
Fernández-Galiano D (1994) The ammoniacal silver carbonate method as a general procedure in the study of protozoa from sewage (and other) waters. Wat Res 28:495–496. doi:10.1016/0043-1354(94)90288-7
Finlay BJ (1980) Temporal and vertical distribution of ciliophoran communities in the benthos of a small eutrophic loch with particular reference to the redox profile. Freshwat Biol 10:15–34
Finlay BJ (1982) Procedures for the isolation, cultivation and identification of protozoa. Exp Microb Ecol 1:44–65
Foissner W (1987) Soil protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Prog Protistol 2:69–112
Foissner W (1996) Terrestial ciliates (Protozoa, Ciliophora) from two islands (Gough, Marion) in the southern oceans, with description of two new species, Arcuospathidium cooperi and Oxytricha ottowi. Archiv für Protistenkol 23:282–291. doi:10.1007/BF00335956
Foissner W, Berger H (1996) A user-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes and waste waters, with notes on their ecology. Freshwat Biol 35:375–470
Gilbert D, Amblard C, Bourdier G, Francez AJ (1998) The microbial loop at the surface of a peatland: structure, functioning and impact of nutrients inputs. Microb Ecol 35:89–93. doi:10.1007/s002489900062
Golterman HL (1969) Methods for chemical analysis of freshwaters. Blackwell Scientific, Oxford
Järvinen M (1993) Pelagic ciliates in acidified mesohumic forest lake before and after lime addition. Ver Internat Verein Limnol 25:534–538
Jerome CA, Montagnes DJS, Taylor FJR (1993) The effect of the quantitative protargol stain and Lugol’s and Buino’s fixatives on cell size: a more accurate estimate of ciliate species biomass. J Euk Microbiol 40:254–259
Kepner RL Jr, Wharton RA Jr (1997) McMurdo LTER: characterization of protozoan communities in lakes Hoare and Fryxell utilizing artificial substrates. Antarct J US 31:201–202
Küppers GC, González GC, Quiroga MV, Lombardo R, Marinone MC, Vinocur A, Mataloni G (2016) Drivers of highly diverse planktonic ciliate assemblages in peat bog pools from Tierra del Fuego (Argentina). Hydrobiologia. doi:10.1007/s10750-016-2686-x
Laybourn-Parry J, Marchant HJ, Brown P (1991) The plankton of a large oligotrophic freshwater Antarctic lake. J Plankton Res 13:1137–1149. doi:10.1093/plankt/13.6.1137
Lee JJ, Small EB, Lynn DH, Bovee EC (1985) Some techniques for collecting, cultivating and observing Protozoa. In: Lee JJ, Hutner SH, Bovee EC (eds) An illustrated guide to the protozoa. Society of protozoologists. Allen, Lawrence, pp 1–7
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. University Press, Cambridge
Marshall WA (1996) Biological particles over Antarctica. Nature 383:680. doi:10.1038/383680a0
Martinov V, Rakusa-Suszczewski S (1989) Ten years of climate observations at the Arctowski and Bellingshausen Stations (King George Island, South Shetlands, Antarctica). In: Breymeyer A (ed) Global Change Regional Research Centres. Scientific problems and concept developments, Warsaw, pp 80–90
Mieczan T (2005) Periphytic ciliates in littoral zone of three lakes of different trophic status. Pol J Ecol 53:489–502
Mieczan T (2007) Seasonal patterns of testate amoebae and ciliates in three peatbogs: relationship to bacteria and flagellates (Poleski National Park, Eastern Poland). Ecohydrol Hydrobiol 1:295–305. doi:10.1016/S1642-3593(07)70191-X
Mieczan T (2009) Ciliates in Sphagnum peatlands: vertical micro-distribution, and relationships of species assemblages with environmental parameters. Zool Stud 48:33–48
Mieczan T, Tarkowska-Kukuryk M (2014) Ecology of moss dwelling ciliates from King George Island (Antarctic): the effect of environmental parameters. Pol Polar Res 4:609–625. doi:10.2478/popore-2014-0026
Mieczan T, Górniak D, Świątecki A, Zdanowski M, Tarkowska-Kukuryk M (2013) The distribution of ciliates on Ecology Glacier (King George Island, Antarctica): relationships between species assemblages and environmental parameters. Polar Biol 36:249–258. doi:10.1007/s00300-012-1256-6
Mueller DR, Vincent WF, Bonilla S, Laurion I (2005) Extremotrophs, extremophiles and broadband pigmentation strategies in a high Arctic Ice Shelf ecosystem. FEMS Microbiol Ecol 53:73–87. doi:10.1016/j.femsee.2004.l
Nijssen D, Rousseau R, Van Hecke P (1998) The Lorenz curve: a graphical representation of evenness. Coenoses 13:33–38
Peeters K, Hodgson DA, Convey P, Willems A (2011) Culturable diversity of heterotrophic bacteria in Forlidas Pond (Pensacola Mountains) and Lundström Lake (Shackleton Range), Antarctica. Microb Ecol 62:399–413. doi:10.1007/s00248-011-9842-7
Petz W (1997) Ecology of the active soil microfauna (Protozoa, Metazoa) of Wilkes Land, East Antarctica. Polar Biol 18:33–44. doi:10.1007/s003000050156
Petz W, Valbonesi A, Schiftner U, Quesada A, Ellis-Evans C (2007) Ciliate biogeography in Antarctic and Arctic freshwater ecosystems: Endemism or global distribution of species? FEMS Microbiol Ecol 59:396–408. doi:10.1111/j.1574-6941.2006.00259.x
Pierce RW, Turner JT (1992) Ecology of plankton ciliates in marine food webs. Rev Aquat Sci 6:139–181
Quesada A, Fernández-Valiente E, Hawes I, Howard-Williams C (2008) Benthic primary production in polar lakes and rivers. In: Laybourn-Parry J, Vincent WF (eds) Limnology of Arctic and Antarctic Aquatic Ecosystems. Oxford University Press, Polar Lakes and Rivers
Roberts EC, Priscu JC, Wolf C, Lyons B, Laybourn-Parry J (2004) The distribution of microplankton in the McMurdo Dry Valley Lakes, Antarctica: Response to ecosystem legacy or present-day climatic control? Polar Biol 27:238–249. doi:10.1007/s00300-003-0582-0
Safi K, Hawens I, Sorrell B (2012) Microbial population responses in three stratified Antarctic meltwater ponds during the autumn freeze. Antarctic Sci 1:1–18. doi:10.1017/S0954102012000636
Sakaeva A, Sokol ER, Kohler TJ, Stanish LF, Spaulding SA, Howkins A, Welch KA, Lyons WB, Barrett JE, McKnight DM (2016) Evidence for dispersal and habitat controls on pond diatom communities from the McMurdo Sound Region of Antarctica. Polar Biol. doi:10.1007/s00300-016-1901-6
Säwström C, Mumford P, Marshall W, Hadson A, Laybourn-Parry J (2002) The microbial communities and primary productivity of cryoconite holes in Arctic glacier (Svalbard 79°N). Polar Biol 25:591–596. doi:10.1007/s00300-002-0388-5
Sutherland DL (2009) Microbial mat communities in response to recent changes in the physicochemical environment of the meltwater ponds on the McMurdo Ice Shelf, Antarctica. Polar Biol 32:1023–1032. doi:10.1007/s0030-009-0601-x
Taton A, Grubisic S, Balthasart P, Hodgson DA, Laybourn-Parry J, Wilmotte A (2006a) Biogeographical distribution and ecological ranges of benthic cyanobacteria in East Antarctic lakes. FEMS Microbiol Ecol 57:272–289. doi:10.1111/j.1574-6941.2006.00110.x
Taton A, Grubisic S, Ertz D, Hodgson DA, Piccardi G, Biondi N, Tredici MR, Mainini M, Losi D, Marinelli F, Wilmotte A (2006b) Polyphasic study of antarctic cyanobacterial strains. J Phycol 42:1257–1270. doi:10.1111/j.1529-8817.2006.00278.x
Utermölh H (1958) Zur vervollkommnung der quantative phytoplankton-methodik. Mitt Int Ver Limnol 9:1–38
Velázquez D, Rochera C, Camacho A, Quesada A (2011) Temperature effects on carbon and nitrogen metabolism in some Maritime Antarctic freshwater phototrophic communities. Polar Biol 34:1045–1055. doi:10.1007/s00300-011-0964-7
Vincent WF, Howard-Williams C (2000) Life on snowball earth. Science 287(5462):2421
Vincent WF, James MR (1996) Biodiversity in extreme aquatic environments: lakes, ponds and streams in the Ross Sea sector, Antarctica. Biodivers Conserv 5:1451–1471
Vinocur A, Pizarro H (2000) Microbial mats of twenty-six lakes from Potter Peninsula, King George Island, Antarctica. Hydrobiologia 437:171–185
Wilkinson DM (2001) What is the upper size limit for cosmopolitan distribution in free-living microorganisms? J Biogeogr 28:258–291. doi:10.1046/j.1365-2699.2001.00518.x
Zhang L, Jungblut AD, Hawes I, Anderson DT, Sumner DY, Mackey TJ (2015) Cyanobacterial diversity in benthic mats of the McMurdo Dry Valley lakes, Antarctica. Polar Biol 38:1097–1110. doi:10.1007/s00300-1669-0
Acknowledgments
We thank our colleagues Dorota Górniak, Aleksander Świątecki, and Adam Latusek for their invaluable help and technical assistance during collection of the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mieczan, T., Adamczuk, M. & Tarkowska-Kukuryk, M. Ecology of ciliates in microbial mats in meltwater streams, King George Island, maritime Antarctica. Polar Biol 40, 1071–1083 (2017). https://doi.org/10.1007/s00300-016-2034-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-2034-7