Abstract
Sea ice in Hudson Bay is melting earlier and freezing later as the climate warms, resulting in declines in the condition, survival, and population size of polar bears (Ursus maritimus) in the Western Hudson Bay population. The objective of this study was to analyse temporal variation in polar bear distribution on the sea ice in Hudson Bay to determine how home range size and location may be responding to changing sea ice conditions and to examine the current population boundary. Between 1990 and 2012, 153 satellite collars were deployed on 141 adult females yielding 67,495 usable locations. We examined annual minimum convex polygons and seasonal utilization distributions. Home ranges in the 1990s (mean = 264,356 ± 30,551 km2) did not differ significantly (t16 = −1.96, P = 0.07) from those in 2004 to 2012 (mean = 353,557 ± 33,719 km2). Home range distribution of individuals differed between seasons and across years, with most variation in the freeze-up and break-up seasons. Home range size was predicted by season, ice break-up date, and individual in a multiple regression, though R 2 was low. Solitary females had smaller home ranges and were closer to land compared to females with offspring. While on the sea ice, the population boundary often encompassed only half of the 2004–2012 polar bear locations and should be reassessed. The distribution of polar bears has shifted both annually and seasonally since 2004, but the consequences remain unclear as the system is extremely variable.
Similar content being viewed by others
Introduction
The distributions and geographic ranges of species are heavily influenced by climate and the spatial patterning of habitat resources (Andrewartha and Birch 1954; Brown 1984; Parmesan and Yohe 2003; Thomas et al. 2004). Recent climate warming coupled with models that project continued warming (Vinnikov et al. 1999; Comiso 2003; Walsh 2008; IPCC 2013) lead to expectations that the ranges of many species will shift as animals seek suitable conditions and appropriate habitat, though the timescale over which these shifts will happen varies by region and species (Thomas and Lennon 1999; Root et al. 2003; Parmesan 2006; Grebmeier 2012). Polar marine habitats will be particularly affected by climate change, and recent warming has been associated with changes in sea level, water temperature, ocean currents, sea ice cover, and marine productivity (Comiso 2002; Parkinson and Cavalieri 2002; Walsh 2008; Palmer et al. 2011). Sea ice is particularly sensitive to climate change, showing reductions in area, thickness, and timing of ice cover (Maslanik et al. 1996; Parkinson 2000; Serreze et al. 2007; Markus et al. 2009); these reductions are expected to continue (Holland et al. 2006; Stroeve et al. 2007; Joly et al. 2010; Castro de la Guardia et al. 2013). Warming temperatures and altered atmospheric circulation have affected the extent, duration, and characteristics of annual sea ice; these changes in sea ice vary geographically (Vinnikov et al. 1999; Lindsay and Zhang 2005; Stroeve et al. 2005) and as such, distributional shifts of animals dependent on sea ice are difficult to predict. Changes in sea ice have affected Arctic marine mammals through habitat loss (Laidre et al. 2008), declining health and condition (Burek et al. 2008), altered prey availability and foraging behaviour (Bluhm and Gradinger 2008), and increased human activities (Hovelsrud et al. 2008). Southern areas where sea ice occurs, such as Hudson Bay, Canada, have experienced particularly significant changes over the past several decades (Gagnon and Gough 2005; Hochheim et al. 2011).
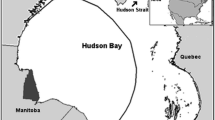
Annual sea ice in Hudson Bay starts to form in November and persists into summer when it melts completely (Prinsenberg 1988). Spring temperatures in this region have increased by 2–3 °C over the last 50 years (Skinner et al. 1998; Gagnon and Gough 2005) and, as a result, the sea ice now breaks up (i.e. reaches ≤50 % cover) approximately 3 weeks earlier than it did 30 years ago (Stirling and Parkinson 2006), affecting sea ice-dependent species such as the polar bear (Ursus maritimus) (see review by Stirling and Derocher 2012). Three populations of polar bears occur in Hudson Bay (Fig. 1): Western Hudson Bay (WH), Southern Hudson Bay (SH), and Foxe Basin (FB). During the ice-free period, these populations are relatively discrete (Peacock et al. 2010; Obbard and Middel 2012); however, the extent to which the current population boundaries reflect their distribution on the sea ice, especially at different times of the year, is not clearly understood.
The 95 % minimum convex polygon (black line and coastline of western Hudson Bay) of all on-ice GPS locations of Western Hudson Bay adult female polar bears (U. maritimus) from 2004 to 2010. Mean sea ice concentrations within this area were used to divide the on-ice period of polar bears into four seasons. Most polar bear captures occurred in Wapusk National Park (shaded area; NE corner at 58°46′N 93°12′W). The dashed line indicates the boundaries for the Western Hudson Bay (WH), Foxe Basin (FB), and Southern Hudson Bay (SH) populations as recognized by the IUCN/SSC Polar Bear Specialist Group (2010)
Polar bears are affected by changing sea ice patterns because they depend on sea ice for hunting, travelling, and some aspects of reproduction (Stirling and Derocher 1993; Ferguson et al. 1999; Amstrup 2003). Polar bears rely on ringed seals (Pusa hispida) and bearded seals (Erignathus barbatus) as their primary prey, which they access while on sea ice (Smith 1980; Thiemann et al. 2008). The earlier break-up of sea ice in spring (Stirling and Parkinson 2006) and progressively later freeze-up in autumn (Hochheim and Barber 2010) increase the length of time that polar bears in Hudson Bay must spend on land by at least several weeks (Stirling et al. 1993; Cherry et al. 2013). These changes were linked to declines in body condition, reproduction, survival, abundance, and increased human–bear conflict in the WH population caused by nutritional stress from reduced energy intake in combination with prolonged fasting (Stirling et al. 1999; Stirling and Parkinson 2006; Regehr et al. 2007; Towns et al. 2009). Changes in sea ice phenology and composition may be affecting the timing of movements made by polar bears in Hudson Bay.
Patterns of ice break-up influence the movement of polar bears as they are returning to shore (Stirling et al. 1999; Cherry et al. 2013). Polar bears in WH show fidelity during summer to terrestrial areas near the Manitoba coast between the Churchill and Nelson rivers (Derocher and Stirling 1990; Stirling et al. 2004). Because the last remaining ice during break-up is adjacent to the coasts of Manitoba and Ontario (Wang et al. 1994; Gough and Allakhverdova 1999; Saucier et al. 2004), WH bears either move onto land when it melts adjacent to Manitoba or extend their hunting time by remaining on ice as it drifts towards the Ontario coast (Stirling et al. 2004). If break-up occurs early off the coast of Manitoba, WH bears may move onto shore earlier than normal, increasing their onshore fasting period. If bears remain on the ice longer and move onshore in Ontario, they often walk back along the coast to Manitoba (Stirling et al. 1999; Cherry et al. 2013) with potential energetic consequences. Hunting and travelling are thought to become less successful as break-up advances (Cherry et al. 2013), and differences in the timing of both break-up and freeze-up across years could affect the distribution of polar bear home ranges, but this remains unexplored.
Considering the recent climatic changes in Hudson Bay and the possible resulting shifts in WH polar bear distribution, it is important to consider if the established geographic population boundary is appropriate. Obbard and Middel (2012) concluded that the established population boundary for the SH polar bear population reflects the current spatial distribution, an important finding for management. The WH and SH populations overlap in the middle of Hudson Bay, but the extent of overlap is unknown. While the genetic consequences are not fully understood, it is likely that gene flow between the three populations is substantial given that mating occurs during spring.
Polar bears are not territorial and have home ranges varying in size depending on seal distribution, year or time of year, sea ice conditions, individual behaviour, and reproductive status (Ferguson et al. 1998; Mauritzen et al. 2001). Energy requirements differ with cub age; thus, female reproductive status (i.e. alone or accompanied by cubs, and the number and age of cubs) may affect distribution and movements (Amstrup et al. 2000; Mauritzen et al. 2001). For a species that only reproduces approximately every 3 years (Ramsay and Stirling 1988), long-term data are necessary for comparing current and past studies. The WH population is the most studied in the world, and one of the most southerly, providing an opportunity to examine the temporal dynamics of distribution. Nonetheless, there has been little investigation of WH polar bear distribution during the on-ice period in the last decade.
In this study, we examine the temporal dynamics of home ranges and distribution of adult female polar bears in the WH population using satellite telemetry. We compare home range size of female polar bears in the 1990s to the 2000s, examine how well the current population boundary reflects WH space use, and measure annual utilization distributions (UDs) in the 2000s as well as seasonal UDs in the 2000s based on reproductive status. We hypothesize that annual and seasonal home range size and distance to land will change and that these changes, along with directional shifts, will be related to changes in sea ice timing associated with shifting climate patterns in the region.
Materials and methods
Hudson Bay is a large (ca. 106 km2) shallow inland sea with a mean depth of 125 m (Jones and Anderson 1994) and a counter clockwise gyre flowing south from Foxe Basin and exiting through Hudson Strait (Prinsenberg 1988). Ice starts forming mid- to late autumn in the northwest and is pushed south by the gyre towards Manitoba and Ontario. From late December to late April, ice cover reaches >9/10. Break-up starts in May as the southernmost ice melts, and ice from the northwest moves south (Maxwell 1986; Saucier et al. 2004) with the last ice occurring off Ontario (Gough et al. 2004). The WH polar bear population is bordered by 63°10′N and 88°30′W and includes coastal regions of Nunavut, Manitoba, and northwestern Ontario (IUCN/SSC Polar Bear Specialist Group 2010) (Fig. 1).
In summer and autumn, Hudson Bay is ice-free, which forces the WH population onshore along the Bay’s western coast (Stirling and Archibald 1977; Stirling et al. 1999). As part of long-term research on the ecology, population dynamics, and status of the WH population, polar bears were captured and collared largely within Wapusk National Park, Manitoba, in August–September 1990–1998 and 2004–2012 (Fig. 1). Bears were located from a helicopter and immobilized via remote injection of tiletamine hydrochloride and zolazepam hydrochloride (Zoletil®, Laboratories Virbac, Carros, France; Stirling et al. 1989). The location, sex, and reproductive status of the bears were recorded. Satellite-linked (CLS Argos, Lanham, MD) Doppler shift (DS) and global positioning system (GPS) collars (Telonics Inc., Mesa, AZ) were deployed on adult females in 1990–1998 and 2004–2012, respectively. The frequency of locations varied depending on research objectives with DS collars programmed to provide locations every 2–10 days and GPS collars every 4 h. Only DS locations with accuracy <1,500 m were used for calculations. The few sudden and far-reaching movements that were biologically impossible (e.g. 8-h round-trips to Russia) were excluded from analyses.
Collars were deployed on solitary adult females (≥5 years old) and females with cubs-of-the-year (cubs) or with 1-year-old cubs (yearlings). Males were not tracked because their necks are wider than their heads, and collars could not be secured. The number of bears collared and transmission intervals varied; thus, sample sizes differ for each analysis. Animal handling procedures were approved by the University of Alberta BioSciences Animal Policy and Welfare Committee and the Animal Care Committee of the Canadian Wildlife Service (Prairie and Northern Region).
Bear locations were plotted as latitude north and longitude west (North American Datum 1983) and converted to universal transverse Mercator coordinates for zone 15 in ArcGIS version 10.0 (Environmental Systems Research Institute Inc., Redlands, CA). ArcGIS was used for all spatial and sea ice analyses unless otherwise stated. Sea ice patterns were examined within a 95 % minimum convex polygon (MCP) calculated from satellite telemetry locations from 2004 to 2010; approximately 56, 000 on-ice locations were used (Fig. 1). We used mean ice concentrations to create 4 seasons: freeze-up (≥1/10 to <9/10 ice cover in the MCP), early winter and late winter (both with ≥9/10 ice cover), and break-up (1/20 to <4/10 ice cover). Early and late winter had the same ice cover, but were split because late winter included mothers who had emerged from their maternity dens with new cubs and coincided with seal pupping and moulting (Ramsay and Andriashek 1986; Amstrup and Gardner 1994). The progression of freeze-up to break-up spans two different years, but we used the year in which freeze-up occurred, referred to as collar year in figures, for the following seasons. We extracted daily ice concentrations from the MCP that were approximated from daily Special Sensor Microwave/Imager (SSM/I) passive microwave data (25 × 25 km resolution) from the National Snow and Ice Data Center (Boulder, CO; Comiso 2000). Dates when each ice concentration was realized were converted to ordinal numbers and averaged to standardize the seasons.
We used several measures of home range size to examine the area occupied by a bear within a given period of time: annual population MCPs, annual population utilization UDs, seasonal population UDs, and individual seasonal UDs. MCP (100 %) home range estimation was chosen to calculate annual population home ranges because it is simple to use with different amounts of data, has been the most commonly used method in other populations, and it facilitates comparisons, especially with Parks et al. (2006). All UDs were calculated at 95 %. UDs more accurately reflect use areas than MCPs method and estimate the intensity or probability of use by an animal throughout its home range, allowing us to calculate home ranges by estimating the area corresponding to any desired probability of use (e.g. 95 % home range area by UD volume; Worton 1989; Millspaugh et al. 2006). We estimated seasonal UDs only if there were ≥20 locations (to compare to Parks et al. 2006) using fixed kernel analysis (Worton 1989) for each GPS-collared bear on the sea ice from 2004 to 2012. Each UD represented a relative probability of use (summing to 1) and was directly comparable across bears. We used the least-squares cross-validation method to determine the smoothing factor (Gitzen et al. 2006) using the KS package (Duong 2011) and adehabitat packages (Calenge 2006) for the R statistical computing software (R Development Core Team, Vienna, Austria).
All locations from 1990s DS data and 2000s GPS data (September 1–August 31) were used for the annual population MCPs. To compare the MCPs from DS data to the more abundant GPS data, we randomly subsampled the GPS locations to match DS location frequency of one location per week per bear. The mean number of locations per year for the older DS collars (all collars combined) was 420 ± 96 (SE) (n = 46 bears). After subsampling to 1 location per week per bear, the mean number of locations per year for GPS collars (all collars combined) was 334 ± 54 (n = 95 bears). The coefficient of variation was calculated for MCPs in 1990–1998 and in 2004–2012.
Annual and seasonal UDs were calculated for the higher-quality on-ice GPS data from 2004 to 2012 to examine space use; DS data often did not meet our requirements for UD calculations, and therefore, only data from the 2000s were used to calculate UDs. Annual population UDs were overlaid to show areas with the highest use from 2004 to 2012. The mean per cent of locations within the WH population boundary (IUCN/SSC Polar Bear Specialist Group 2010) was also calculated by year and season from 2004 to 2012. Seasonal individual UDs were grouped by reproductive class for some analyses. Females collared with cubs in autumn moved to “females with yearlings” for the following late winter and break-up seasons. A female collared with yearlings in September was considered to have yearlings for that freeze-up and early winter, but was of “unknown” status for that late winter and break-up seasons. A solitary female collared in September that denned and emerged with cubs, based on location data, was classified as having cubs in that late winter and break-up seasons. Females with unknown cub status 1 year after capture were “unknown reproductive status”. While female status was updated if she was located in the field 1–2 years after collaring, there is a chance that some females have been given the wrong classification in certain analyses due to our assumptions of a 3-year reproductive cycle.
To determine seasonal distribution, centroids were calculated for each seasonal individual UD, and we measured their distance to the nearest land on the west coast of Hudson Bay (from western James Bay to Chesterfield Inlet). Values of 0 km from the coast in early winter were removed as they were likely due to maternity denning; values of 0 during freeze-up and break-up were included because ice conditions could cause use of land.
All linear statistics were performed with SPSS® version 21.0 for Windows (SPSS Inc., Chicago, IL). We tested the null hypothesis that MCP size was independent of decade using a t test, and the coefficient of variation for each decade was calculated. For individual UD size and centroid distance to land analyses, we tested the null hypothesis that measured variables were independent of season or reproductive class using one-way ANOVAs when data were normal and had equal variance. Non-normal data were log10 transformed but if heteroskedasticity remained, we used the Kruskal–Wallis H or Mann–Whitney U nonparametric tests (Sokal and Rohlf 2001). ANOVAs and Kruskal–Wallis tests were followed by either Bonferroni test or nonparametric Tukey’s test (Zar 1999) to determine significant differences. Additionally, we examined correlations between range sizes in successive seasons with either Pearson’s product-moment correlation (coefficient reported as r) for normal data or the Spearman’s rank-order correlation (coefficient reported as r s ) for heteroskedastic data. Coefficients of variation (CV) in home range size were calculated for each season from 2004 to 2012. We ran multiple regressions to test the null hypothesis that individual UD size and centroid distance to land were independent from other variables including individual, year, reproductive class, season, and ice events such as the date of break-up (when ice cover reached ≤4/10 in the MCP) and the date of freeze-up (when ice cover reached ≥1/10 in the MCP). For all analyses, values are means ± 1 SE. Results were considered significant at P ≤ 0.05.
Seasonal population UDs were calculated after pooling all animals by year and season. A centroid was calculated for each seasonal population UD and, following Zar (1999), vectors (mean direction and orientation) were calculated between centroids in concurrent years within the same season for 2004–2012. For each season, we calculated and mapped the mean direction, directional variance, distance, and geographic centre of centroid shift using north as 0°. Circular statistics were performed with Oriana© version 4.02 (Kovach Computing Services, Anglesey, WLS).
Results
DS collars were deployed on 46 females in 1990–1998, and GPS collars on 95 females in 2004–2012 (11 bears were collared twice and 1 bear was collared three times). For DS collars, 3,781 locations were analysed totalling 63 bear years (17 bears had usable data for more than 1 year). For GPS collars, 63,714 locations of approximately 108,000 were analysed, totalling 109 bear years and 297 bear seasons (24 bears had usable data for more than 1 year; 22 collars failed prematurely). The number of GPS locations varied by season: 7,684 for freeze-up, 25,338 for early winter, 29,479 for late winter, and 1,215 for break-up. The four on-ice seasons examined were freeze-up (November 27–December 18, 22 days), early winter (December 19–March 14, 86 days), late winter (March 15–July 1, 109 days), and break-up (July 2–14, 13 days).
MCPs were normally distributed and not significantly correlated with year (r = 0.35, P = 0.16). The mean annual MCP size for 1990–1998 (264,356 ± 30,551 km2, n = 9; CV = 0.35) was not significantly different (t 16 = −1.96, P = 0.07) from 2004 to 2012 (353,557 ± 33,719 km2, n = 9; CV = 0.29) (Fig. 2). Pooled annual population UDs in 2004–2012 revealed substantial variation in use areas (Fig. 3). Use of the most eastern parts of Hudson Bay was limited. The per cent of locations that fell within the WH population boundary varied by season and year: freeze-up and break-up had the highest mean per cent of locations within the WH boundary, and early winter had the lowest (Table 1). By year, 2005 had the highest mean per cent of locations within the boundary, while 2004 and 2006 had the lowest.
Annual minimum convex polygon (MCP) area (1,000 km2) ± SE for all locations per year from collared adult female polar bears (U. maritimus) from the Western Hudson Bay population. Data include both land and ice locations; years run from September 1 to August 31. Data from the 1990s are from satellite collars and 2000 data are from GPS collars that were rarefied to 1 location per week per bear to be comparable to the satellite collar data. The number of collars contributing locations to each MCP is indicated above each data point
Annual 95 % utilization distributions for adult female polar bears (U. maritimus) (n = 81) in the Western Hudson Bay population, 2004–2012 (bordered by 63°10′N and 88°30′W). Darker grey areas show more overlap of utilization distributions, while lighter grey areas show less overlap. The solid lines indicate the boundaries for the Western Hudson Bay (WH), Foxe Basin (FB), and Southern Hudson Bay (SH) populations as recognized by the IUCN/SSC Polar Bear Specialist Group (2010)
Seasonal individual UD size varied significantly by season [Kruskal–Wallis, H(3) = 79.01, P < 0.001] (Fig. 4), with the largest UDs occurring during freeze-up (33,604 km2 ± 4,081, n = 76), followed by late winter (31,292 km2 ± 2,290, n = 91) and early winter (30,824 km2 ± 2,326, n = 91), with break-up having the smallest individual UDs (1,548 km2 ± 296, n = 38). Individual UD sizes in successive seasons were not correlated with each other (freeze-up and early winter: r s = −0.06, P = 0.62; early winter and late winter: r s = 0.04, P = 0.71; late winter and break-up: r s = −0.22, P = 0.19). Break-up had the highest coefficient of variation (CV = 1.18) followed by freeze-up (CV = 1.06), then early winter (CV = 0.72), and late winter with the least variation (CV = 0.70). With all seasons grouped, UD size varied by reproductive class with females with cubs having the largest UDs [Kruskal–Wallis, H(2) = 13.09, P = 0.001]. However, within seasons, the only significant difference between reproductive classes was during break-up where females with yearlings had larger UDs than solitary/unknown females (Mann–Whitney U test, U = 93.5, P = 0.03) (Table 2). A multiple regression of seasonal individual UD size with individual, year, season, reproductive class, ice freeze-up date, and ice break-up date was significant [F (6,289) = 8.23, P < 0.001, R 2 = 0.15] with three significant predictors (season: β = −0.43, P < 0.001, break-up date: β = 0.29, P < 0.001, and individual: β = 0.14, P = 0.02). Individual UD size was correlated with centroid distance to land (r s = 0.24, P < 0.001).
Seasonal centroid distance to land varied significantly by season [Kruskal–Wallis, H(3) = 77.35, P < 0.001] (Fig. 5), with the largest distances to land occurring during early winter (202 km ± 7, n = 85), followed by late winter (142 km ± 8, n = 91), freeze-up (105 km ± 9, n = 76), and break-up (72 km ± 9, n = 38). Break-up had the highest coefficient of variation (CV = 0.76) followed by freeze-up (CV = 0.72), then late winter (CV = 0.51), and early winter with the least variation (CV = 0.45). Distances to land in successive seasons were not correlated with each other (freeze-up and early winter: r s = −0.12, P = 0.33; early winter and late winter: r s = 0.09, P = 0.44; late winter and break-up: r s = −0.15, P = 0.39). When all seasons were grouped, distance to land varied significantly by reproductive class with solitary females being closer to land [Kruskal–Wallis, H(2) = 11.37, P < 0.001] (Table 3), but within seasons there were no significant differences among reproductive classes. A multiple regression to predict centroid distance to land from the variables individual, year, season, reproductive class, ice freeze-up date, and ice break-up date was not significant [F (6,289) = 0.96, P = 0.45, R 2 = 0.02].
Seasonal population centroids shifted each year by various magnitudes and directions in each season, though not significantly (Fig. 6). The mean direction of freeze-up centroids shift was 254° (Rayleigh’s z = 0.23, P = 0.81, n = 8), early winter shift was 348° (Rayleigh’s z = 0.62, P = 0.55, n = 8), late winter shift was 312° (Rayleigh’s z = 0.15, P = 0.87, n = 8), and break-up shift was 322° (Rayleigh’s z = 0.16, P = 0.86, n = 7).
Discussion
Polar bears live under different conditions in different parts of the Arctic, and understanding these differences improves our knowledge of how this species may respond to habitat changes. Home range sizes of polar bears are highly variable within and between populations, which may be a result of prey availability, seasonal cycles, and climate. Large home ranges were reported in the Beaufort Sea (up to 596,800 km2; Amstrup et al. 2000) and in the Canadian Archipelago (to 540,700 km2; Ferguson et al. 1999). In the Barents Sea, home ranges were somewhat smaller but extremely variable (185–373,539 km2; Mauritzen et al. 2001). Home range sizes are influenced by the availability and predictability of prey (Ferguson et al. 1999; Mauritzen et al. 2003) and are larger where they encompass considerable amounts of multiyear ice or land (Stirling and Øritsland 1995; Ferguson et al. 1999) and smaller in areas with annual ice and shallow water, such as in Hudson Bay. Polar bear space use may also be affected by the type of sea ice: environments with active ice may increase passive movements due to sea ice drift thereby increasing home range size, while environments with consolidated ice (e.g. fjords) may lead to polar bears having reduced home range sizes simply due to the stable nature of the habitat (Mauritzen et al. 2001). Ringed seals, the polar bear’s main prey, prefer more stable ice and shallower water (Kingsley et al. 1985; Gjertz et al. 2000), and may occur at higher densities in western Hudson Bay compared to other areas of the Arctic (Lunn et al. 1997).
Parks et al. (2006) estimated annual MCP home range of WH polar bears with DS satellite data from the 1990s and found that movement patterns were not dependent on reproductive status but did change with season. Also, they found a decreased home range size from 1991 to 1998 that was correlated with earlier break-up and suggested that the decline in polar bear condition was due to decreased prey availability and not energy expenditure. Our MCPs indicate that the 1990s decline in home range size did not continue into the 2000s. Furthermore, our annual UDs indicate that while some areas are used consistently by WH bears each year, other areas farther north or east are used infrequently. Fidelity of females to the western Hudson Bay denning area is likely an important factor in use areas. While our study focuses on adult females, we assume that this pattern of area use would be similar for males and subadults. The areas used by the population may be affected by changes in ice cover or may be a response to changes in distribution of their prey. In the 1990s, environmental conditions were unfavourable for ringed seals, but improved in the 2000s with three times as many pups than in the early 1990s (Ferguson et al. 2005; Chambellant et al. 2012). While a healthier seal population was evident in the 2000s (Chambellant et al. 2012), polar bear body condition was lower during this time compared to the early 1990s, likely related to the decreased time polar bears had to hunt them (Stirling et al. 1993, 1999; Derocher et al. 2004; Stirling and Parkinson 2006; Regehr et al. 2007). While we recorded variation in annual home range sizes, there were larger variations in the size and location of seasonal home range sizes, most notably during the transition seasons of freeze-up and break-up.
Large movements occur when polar bears move onto the ice to hunt (Stirling and Archibald 1977; Derocher and Stirling 1990; Parks et al. 2006), as they follow the expanding ice edge during freeze-up. Such far-ranging movements may explain why the largest individual seasonal home ranges sizes occurred during freeze-up. If the ice edge expands at different times or rates in different years, it could change how and where polar bears travel, which may also partially explain the variability in the proportion of locations that occurred outside of the WH population boundary. Though directional vectors of centroids were not significant, there was a suggestion of a general shift northwards in all seasons from 2004 to 2012 except during freeze-up where the centroids were closer to Wapusk National Park where the bears spend the ice-free period. This tendency may be related to the fewer offshore locations during the defined freeze-up season related to the delay in sea ice formation (Stirling and Parkinson 2006). Such directional analyses, however, have low statistical power and will require continued monitoring.
The higher variances in home range size and centroid distances during freeze-up and break-up compared to lower variances in early and late winter suggest that individuals make different movement choices, especially when their habitat is changing quickly. However, habitat availability is dynamic during these periods and may change by year, with slow-forming sea ice in some years and fast-consolidating sea ice in other years. As such polar bears may not necessarily make different choices, their movements simply reflect responses to the presence of suitable habitat. WH females may stay on the ice longer (Cherry et al. 2013), even if their ice habitat is less suitable, to deposit fat stores, but such patterns may vary by individual. This could be why home range size was explained partly by the variables of break-up date and individual. Whether a female stays on the ice longer to hunt or moves to land when the ice starts to break up may be influenced by her reproductive status and energetic demands.
Females may adjust their movements based on the presence of cubs. Due to their small size, young cubs are vulnerable to hypothermia (Blix and Lentfer 1979) and cannibalism by male polar bears (Taylor et al. 1985; Amstrup et al. 2006); females with cubs may adjust their spatial distribution to reduce contact with adult males (Derocher and Stirling 1990; Stirling et al. 1993). In our study, reproductive status influenced home range size and distribution during break-up, with solitary females having smaller ranges and staying closer to land than females with offspring. A different result was found in Svalbard where females with young cubs had smaller spring home ranges than females with older offspring or solitary females, but this was explained by the need for females with vulnerable young cubs to stay in a stable habitat and therefore avoid the unstable and drifting ice further from land (Freitas et al. 2012). Svalbard differs from the Hudson Bay system in that Hudson Bay is surrounded by land and therefore may be more stable for mothers with cubs. Thus, WH females with cubs can travel farther from land more safely. Females with young can have daily energy expenditures of approximately four times their basal metabolic rate due to lactation and food-sharing (Ricklefs et al. 1996), causing them to feed more than other females. Energetic demands in late winter and break-up may cause female polar bears with young to search for prey over larger areas. Females with cubs are arguably the most important demographic in a population, and thus any changes in the environment that affect their distribution could affect the population.
Polar bears now return to the ice later than several decades ago (Stirling and Parkinson 2006; Cherry et al. 2013), causing additional fasting with negative consequences for the population (Stirling et al. 1999; Regehr et al. 2007; Molnár et al. 2010, 2011, 2014). If the ice-free period begins earlier, polar bears have less time to accumulate fat; thus, the timing of the shift to ice-free conditions is critical. An estimated decrease in the WH population from 1194 to 935 bears (22 %) (1984–2004) was related to the earlier break-up of sea ice (Regehr et al. 2007). As the timing of sea ice continues to shift (Castro de la Guardia et al. 2013), it will be important for managers to be aware of the distribution of Hudson Bay’s polar bear populations. From 2004 to 2012, the WH population boundary encompassed less than half of the total locations across seasons, particularly during early winter. Given the substantial number of locations outside of the recognized population boundary, the WH population boundaries should be revisited for management purposes and reconsidered for ecological investigations. As habitat availability changes, so will polar bear distribution; thus, these data series are important and continued monitoring is critical.
References
Amstrup SC (2003) Polar bear. In: Feldhammer GA, Thompson BC, Chapman JA (eds) Wild mammals of North America: biology, management, and conservation. John Hopkins University Press, Baltimore, pp 587–610
Amstrup SC, Gardner C (1994) Polar bear maternity denning in the Beaufort Sea. J Wildl Manage 58:1–10
Amstrup SC, Durner GM, Stirling I, Lunn NJ, Messier F (2000) Movements and distribution of polar bears in the Beaufort Sea. Can J Zool 78:948–966
Amstrup SC, Stirling I, Smith TS, Perham C, Thiemann GW (2006) Recent observations of intraspecific predation and cannibalism among polar bears in the southern Beaufort Sea. Polar Biol 29:997–1002
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Blix AS, Lentfer JW (1979) Modes of thermal protection in polar bear cubs—at birth and on emergence from the den. Am J Physiol 236:R67–R74
Bluhm BA, Gradinger R (2008) Regional variability in food availability for arctic marine mammals. Ecol Appl 18:S77–S96
Brown JH (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279
Burek KA, Gulland FMD, O’Hara TM (2008) Effects of climate change on Arctic marine mammal health. Ecol Appl 18:S126–S134
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Castro de la Guardia L, Derocher AE, Myers PG, Terwisscha van Scheltinga AD, Lunn NJ (2013) Future sea ice conditions in western Hudson Bay and consequences for polar bears in the 21st century. Glob Change Biol 19:2675–2687
Chambellant M, Stirling I, Gough WA, Ferguson SH (2012) Temporal variations in Hudson Bay ringed seal (Phoca hispida) life-history parameters in relation to environment. J Mamm 93:267–281
Cherry SG, Derocher AE, Thiemann GW, Lunn NJ (2013) Migration phenology and seasonal fidelity of an Arctic marine predator in relation to sea ice dynamics. J Anim Ecol 82:912–921
Comiso JC (2000, updated 2014) Bootstrap sea ice concentrations from Nimbus-7 SMMR and DMSP SSM/I-SSMIS. Version 2. [2004–2012]. NASA DAAC at the National Snow and Ice Data Center, Boulder
Comiso JC (2002) A rapidly declining perennial sea ice cover in the Arctic. Geophys Res Lett 29:1956–1959
Comiso JC (2003) Warming trends in the Arctic from clear sky satellite observations. J Clim 16:3498–3510
Derocher AE, Stirling I (1990) Distribution of polar bears (Ursus maritimus) during the ice-free period in western Hudson Bay. Can J Zool 68:1395–1402
Derocher AE, Lunn NJ, Stirling I (2004) Polar bears in a warming climate. Integr Comp Biol 44:163–176
Duong T (2011) ks: Kernel smoothing. R package version 1.8.1
Ferguson SH, Taylor MK, Born EW, Rosing-Asvid A, Messier F (1998) Sea-ice landscape and spatial patterns of polar bears. J Biogeogr 25:1081–1092
Ferguson SH, Taylor MK, Born EW, Rosing-Asvid A, Messier F (1999) Determinants of home range size for polar bears (Ursus maritimus). Ecol Lett 2:311–318
Ferguson SH, Stirling I, McLoughlin P (2005) Climate change and ringed seal (Phoca hispida) recruitment in Hudson Bay. Mar Mamm Sci 21:121–135
Freitas C, Kovacs KM, Andersen M, Aars J, Sandven S, Mauritzen M, Pavlova O, Lydersen C (2012) Importance of fast ice and glacier fronts for female polar bears and their cubs during spring in Svalbard, Norway. Mar Ecol Prog Ser 447:289–304
Gagnon AS, Gough WA (2005) Trends in dates of ice freeze-up and breakup over Hudson Bay, Canada. Arctic 58:370–382
Gitzen RA, Millspaugh JJ, Kernohan BJ (2006) Bandwidth selection for fixed-kernel analysis of animal utilization distributions. J Wildl Manage 70:1334–1344
Gjertz I, Kovacs KM, Lydersen C, Wiig Ø (2000) Movements and diving of bearded seal (Erignathus barbatus) mothers and pups during lactation and post-weaning. Polar Biol 23:559–566
Gough WA, Allakhverdova T (1999) Limitations of using a coarse resolution model to assess the impact of climate change on sea ice in Hudson Bay. Can Geogr 43:415–422
Gough WA, Cornwell AR, Tsuji LJS (2004) Trends in seasonal sea ice duration in southwestern Hudson Bay. Arctic 57:299–305
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and Sub-Arctic seas. Annu Rev Mar Sci 4:63–78
Hochheim KP, Barber DG (2010) Atmospheric forcing of sea ice in Hudson Bay during the fall period, 1980–2005. J Geophys Res Oceans 115:C05009
Hochheim KP, Lukovich JV, Barber D (2011) Atmospheric forcing of sea ice in Hudson Bay during the spring period, 1980–2005. J Mar Syst 88:476–487
Holland MM, Bitz CM, Tremblay B (2006) Future abrupt reductions in the summer Arctic sea ice. Geophys Res Lett 33:L23503
Hovelsrud GK, McKenna M, Huntington HP (2008) Marine mammal harvests and other interactions with humans. Ecol Appl 18:S135–S147
IPCC (Intergovernmental Panel on Climate Change) (2013) Climate change 2013: the physical science basis. Working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
IUCN/SSC Polar Bear Specialist Group (2010) Polar bears: proceedings of the 15th working meeting of the IUCN/SSC Polar Bear Specialist Group. IUCN, Gland, Switzerland and Cambridge
Joly S, Senneville S, Caya D, Saucier FJ (2010) Sensitivity of Hudson Bay sea ice and ocean climate to atmospheric temperature forcing. Clim Dyn 36:1835–1849
Jones EP, Anderson JG (1994) Northern Hudson Bay and Foxe Basin: water masses, circulation and productivity. Atmos Ocean 32:361–374
Kingsley MCS, Stirling I, Calvert W (1985) The distribution and abundance of seals in the Canadian high Arctic, 1980–1982. Can J Fish Aquat Sci 42:1189–1210
Laidre KL, Stirling I, Lowry LF, Wiig Ø, Heide-Jorgensen MP, Ferguson SH (2008) Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol Appl 18:S97–S125
Lindsay RW, Zhang J (2005) The thinning of Arctic sea ice, 1988–2003: Have we passed a tipping point? J Clim 18:4879–4894
Lunn NJ, Stirling I, Andriashek D, Kolenosky GB (1997) Re-estimating the size of the polar bear population in western Hudson Bay. Arctic 50:234–240
Markus MT, Stroeve JC, Miller J (2009) Recent changes in Arctic sea ice melt onset, freezeup, and melt season length. J Geophys Res Oceans 114:C12024
Maslanik JA, Serreze MC, Barry RG (1996) Recent decreases in Arctic summer ice cover and linkages to atmospheric circulation anomalies. Geophys Res Lett 23:1677–1680
Mauritzen M, Derocher AE, Wiig Ø (2001) Space-use strategies of female polar bears in a dynamic sea ice habitat. Can J Zool 79:1704–1713
Mauritzen M, Belikov SE, Boltunov AN, Derocher AE, Hansen E, Ims RA, Wiig Ø, Yoccoz N (2003) Functional responses in polar bear habitat selection. Oikos 100:112–124
Maxwell JB (1986) A climate overview of the Canadian Inland Seas. In: Martini IP (ed) Canadian inland seas, Elsevier oceanography series 44. Elsevier Science, Amsterdam, pp 79–99
Millspaugh JJ, Nielson RM, McDonald L, Marzluff JM, Gitzen RA, Rittenhouse CD, Hubbard MW, Sheriff SL (2006) Analysis of resource selection using utilization distributions. J Wildl Manage 70:384–395
Molnár PK, Derocher AE, Thiemann GW, Lewis MA (2010) Predicting survival, reproduction and abundance of polar bears under climate change. Biol Conserv 143:1612–1622
Molnár PK, Derocher AE, Klanjscek T, Lewis MA (2011) Predicting climate change impacts on polar bear litter size. Nat Commun 2:186
Molnár PK, Derocher AE, Thiemann GW, Lewis MA (2014) Corrigendum to “Predicting survival, reproduction and abundance of polar bears under climate change” [Biol Conserv 143 (2010) 1612–1622]. Biol Conserv 177:230–231
Obbard ME, Middel KR (2012) Bounding the Southern Hudson Bay polar bear subpopulation. Ursus 23:134–144
Palmer MA, Arrigo KR, Mundy CJ, Ehn JK, Gosselin M, Barber DG, Martin J, Alou E, Roy S, Tremblay J-É (2011) Spatial and temporal variation of photosynthetic parameters in natural phytoplankton assemblages in the Beaufort Sea, Canadian Arctic. Polar Biol 34:1915–1928
Parkinson CL (2000) Variability of Arctic sea ice: the view from space, an 18-year record. Arctic 53:341–358
Parkinson CL, Cavalieri DJ (2002) A 21-year record of Arctic sea-ice extents and their regional, seasonal and monthly variability and trends. Ann Glaciol 34:441–446
Parks EK, Derocher AE, Lunn NJ (2006) Seasonal and annual movement patterns of polar bears on the sea ice of Hudson Bay. Can J Zool 84:1281–1294
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Peacock E, Derocher AE, Lunn NJ, Obbard ME (2010) Polar bear ecology and management in Hudson Bay in the face of climate change. In: Ferguson SH, Loseto LL, Mallory ML (eds) A little less arctic: top predators in the world’s largest Northern Inland Sea, Hudson Bay. Springer, Dordrecht, pp 93–116
Prinsenberg SJ (1988) Ice-cover and ice-ridge contributions to the freshwater contents of Hudson Bay and Foxe Basin. Arctic 41:6–11
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramsay MA, Andriashek D (1986) Long-distance route orientation of female polar bears (Ursus maritimus) in spring. J Zool 208:63–72
Ramsay MA, Stirling I (1988) Reproductive biology and ecology of female polar bears (Ursus maritimus). J Zool 214:601–634
Regehr EV, Lunn NJ, Amstrup SC, Stirling I (2007) Effects of earlier sea ice breakup on survival and population size of polar bears in Western Hudson Bay. J Wildl Manage 71:2673–2683
Ricklefs RE, Konarzewski M, Daan S (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1071
Root TL, Price JT, Hall KR, Schneider S, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Saucier FJ, Senneville S, Prisenberg S, Roy F, Smith G, Gachon P, Caya D, Laprise R (2004) Modelling the sea ice-ocean seasonal cycle in Hudson Bay, Foxe Basin, and Hudson Strait, Canada. Clim Dyn 23:303–326
Serreze MC, Holland MM, Stroeve J (2007) Perspectives on the Arctic’s shrinking ice cover. Science 315:1533–1536
Skinner WR, Jeffries RL, Carleton TJ, Rockwell RF, Abraham KF (1998) Prediction of reproductive success and failure in lesser snow geese based on early season climatic variables. Glob Chang Biol 4:3–16
Smith TG (1980) Polar bear predation of ringed seals and bearded seals in the land-fast sea ice habitat. Can J Zool 58:2201–2209
Sokal RR, Rohlf FJ (2001) Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Company, NY
SPSS Inc (2013) SPSS® for Windows. Version 21.0 [computer program]. SPSS Inc., Chicago
Stirling I, Archibald WR (1977) Aspect of predation of seals by polar bears. J Fish Res Board Can 34:1126–1129
Stirling I, Derocher AE (1993) Possible impacts of climatic warming on polar bears. Arctic 46:240–245
Stirling I, Derocher AE (2012) Effects of climate warming on polar bears: a review of the evidence. Glob Chang Biol 18:2694–2706
Stirling I, Øritsland NA (1995) Relationships between estimates of ringed seal (Phoca hispida) and polar bear (Ursus maritimus) populations in the Canadian Arctic. Can J Fish Aquat Sci 52:2594–2612
Stirling I, Parkinson CL (2006) Possible effects of climate warming on selected populations of polar bears (Ursus maritimus) in the Canadian arctic. Arctic 59:261–275
Stirling I, Spencer C, Andriashek D (1989) Immobilization of polar bears (Ursus maritimus) with Telazol® in the Canadian Arctic. J Wildl Dis 25:159–168
Stirling I, Andriashek D, Calvert W (1993) Habitat preferences of polar bears in the western Canadian Arctic in late winter and spring. Polar Rec 29:13–24
Stirling I, Lunn NJ, Iacozza J (1999) Long-term trends in the population ecology of polar bears in western Hudson Bay in relation to climatic change. Arctic 52:294–306
Stirling I, Lunn NJ, Iacozza J, Elliott C, Obbard M (2004) Polar bear distribution and abundance on the southwestern Hudson Bay coast during open water season, in relation to population trends and annual ice patterns. Arctic 57:15–26
Stroeve JC, Serreze MC, Fetterer F, Arbetter T, Meier W, Maslanik J, Knowles K (2005) Tracking the Arctic’s shrinking ice cover: another extreme September minimum in 2004. Geophys Res Lett 32:L04501
Stroeve JC, Holland MM, Meier W, Scambos T, Serreze M (2007) Arctic sea ice decline: faster than forecast. Geophys Res Lett 34:L09501
Taylor M, Larsen T, Schweinsburg RE (1985) Observations of intraspecific aggression and cannibalism in polar bears (Ursus maritimus). Arctic 38:303–309
Thiemann GW, Iverson SJ, Stirling I (2008) Polar bear diets and Arctic marine food webs: insights from fatty acid analysis. Ecol Monogr 78:591–613
Thomas CD, Lennon JJ (1999) Birds extend their ranges northwards. Nature 399:213
Thomas CD, Cameron A, Green RE et al (2004) Extinction risk from climate change. Nature 427:145–148
Towns L, Derocher AE, Stirling I, Lunn NJ, Hedman D (2009) Spatial and temporal patterns of problem polar bears in Churchill, Manitoba. Polar Biol 32:1529–1537
Vinnikov KY, Robock A, Stouffer RJ, Walsh JE, Parkinson CL, Cavalieri DJ, Mitchell JFB, Garrett D, Zakharov VF (1999) Global warming and Northern Hemisphere sea ice extent. Science 286:1934–1937
Walsh JE (2008) Climate of the Arctic marine environment. Ecol Appl 18:S3–S22
Wang J, Mysak LA, Ingram RG (1994) A numerical simulation of sea ice cover in Hudson Bay. J Phys Oceangr 24:2515–2533
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Zar J (1999) Biostatistical analysis, 4th edn. Prentice-Hall Inc., Upper Saddle River
Acknowledgments
Many pilots were involved in safely flying researchers and providing field assistance, but a special thanks to Hudson Bay Helicopters. We also thank the Churchill Northern Studies Centre for accommodation and field support. Funding and logistical support was provided by Aquarium du Québec, ArcticNet, Canadian Association of Zoos and Aquariums, Canadian Circumpolar Institute, Canadian Wildlife Federation, Care for the Wild International, Environment Canada, EnviroNorth, Hauser Bears, the Isdell Family Foundation, Manitoba Conservation, Natural Sciences and Engineering Research Council of Canada, Northern Science Training Program, Parks Canada, Polar Bears International, Quark Expeditions, the University of Alberta, W. Garfield Weston Foundation, Wildlife Media Inc., and World Wildlife Fund (Canada).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCall, A.G., Derocher, A.E. & Lunn, N.J. Home range distribution of polar bears in western Hudson Bay. Polar Biol 38, 343–355 (2015). https://doi.org/10.1007/s00300-014-1590-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1590-y