Abstract
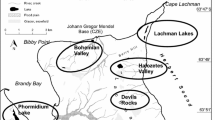
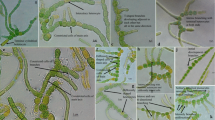
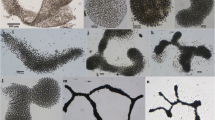
Several communities of autotrophic microorganisms, in which cyanobacteria are dominant or play a substantial role in their structure, were studied on the deglaciated Ulu Peninsula, northern part of James Ross Island, NW Weddell Sea, Antarctica, in 2007–2009. Our results were compared with similar data from maritime Antarctica (King George Island, South Shetland Islands, 2005). Characteristics and taxonomic description of three important heterocytous species, which participate in cyanoprokaryotic assemblages in the littoral of small lakes, seepages, and on wetted rocks during the Antarctic summer season, are included in this study. They belong to the form-genera Calothrix and Hassallia, respectively, and are unidentifiable according to the present determination literature. Therefore, after a polyphasic evaluation, they are described as three new species, Calothrix elsteri sp. nova, Hassallia andreassenii sp. nova, and Hassallia antarctica sp. nova. 16S rRNA gene sequencing of isolated strains confirmed the taxonomic position of all three species, and their ecology and seasonal development are described. All three discovered species are dominant in distinct communities with a specialized ecology and may be endemic for coastal maritime Antarctica.










Similar content being viewed by others
References
Akiyama M (1968) A list of terrestrial and subterranean algae from the Ongul Islands, Antarctica. Antarct Rec 32:71–77
Boyer SL, Johansen JR, Flechtner VR, Howard GL (2002) Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S–23S its region. J Phycol 38:1222–1235
Broady PA (1986) Ecology and taxonomy of the terrestrial algae of the Vestfold Hills. In: Pickard J (ed) Antarctic Oasis: terrestrial environments and history of the Vestfold Hills, chapter 6. Academic Press, Australia, pp 165–202
Broady PA (1989) Survey of algae and other terrestrial biota at Edward VII Peninsula, Marie Byrd Land. Antarct Sci 1:215–224
Broady PA (1996) Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodivers Conserv 5:1307–1335
Broady PA, Kibblewhite AL (1991) Morphological characterization of Oscillatoriales (Cyanobacteria) from Ross-Island and Southern Victoria Land, Antarctica. Antarct Sci 3:35–45
Broady PA, Smith RA (1994) A preliminary investigation of the diversity, survivability and dispersal of algae introduced into Antarctica by human activity. Proc NIPR Symp Polar Biol 7:185–197
Broady PA, Given D, Greenfield L, Thompson K (1987) The biota and environment of fumaroles on Mt Melbourn, Northern Victoria Land. Polar Biol 7:97–113
Casamatta DA, Johansen JR, Vis ML, Broadwater ST (2005) Molecular and morphological characterization of ten polar and near-polar strains within the Oscillatoriales (Cyanobacteria). J Phycol 41:421–438
Castenholz RW (1992) Species usage, concept, and evolution in the cyanobacteria (blue-green algae). J Phycol 28:737–745
Czech Geological Survey (2009) James Ross Island—Northern Part. Topographic map 1:25000, CGS, Praha
Flechtner VR, Boyer SL, Johansen JR, DeNoble ML (2002) Spirirestis rafaelensis gen. et sp. nov. (Cyanophyceae), a new cyanobacterial genus from arid soils. Nova Hedwigia 74:1–24
Fritsch FE (1912) Freshwater algae. National Antarctic “Discovery” Expedition 1901–1904. British Mus Nat Hist 6:1–66
Garcia-Pichel F (2008) Molecular ecology and environmental genomics of Cyanobacteria. In: Herrero A, Flores E (eds) The Cyanobacteria. Molecular biology, genomics and evolution. Caister Academic Press, Norfolk, pp 59–87
Garcia-Pichel F, Nübel U, Muyzer G (1998) The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch Microbiol 169:469–482
Gardel RW, Drouet F (1960) The cyanobacteria of the Thule area, Greenland. Trans Am Microsc Soc 79:256–272
Gordon DA, Priscu JC, Giovannoni S (2000) Origin and phylogeny of microbes living in permanent Antarctic lake ice. Microb Ecol 39:197–202
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hirano M (1965) Freshwater algae in the Antarctic region. In: van Mieghem J, van Oye P, Schell J (eds) Biogeography and ecology in Antarctica, vol. 15. Monogr Biol, pp 127–193
Hjort C, Ingólfsson Ó, Möller P, Lirio JM (1997) Holocene glacial history and sea-level changes on James Ross Island, Antarctic Peninsula. J Quart Sci 12:259–273
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Johansen JR, Casamatta DA (2005) Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algolog Stud 117:71–93
Jungblut AD, Hawes I, Mountfort D, Hitzfeld B, Dietrich DR, Burns BP, Neilan BA (2005) Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ Microbiol 7:519–529
Jungblut AD, Lovejoy C, Vincent WF (2010) Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J 4:191–202
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Komárek J (1999) Diversity of cyanoprokaryotes (cyanobacteria) of King George Island, maritime Antarctica—a survey. Algolog Stud 94:181–193
Komárek J (2010) Recent changes (2008) in cyanobacterial taxonomy based on a combination of molecular background with phenotype and ecological consequences (genus and species concept). Hydrobiologia 639:245–259
Komárek J (2011) Introduction to the 18th IAC Symposium in České Budějovice 2010, Czech Republic. Some current problems of modern cyanobacterial taxonomy. Fottea 11:1–7
Komárek J, Elster J (2008) Ecological background of cyanobacterial assemblages of the northern part of James Ross Island, NW Weddell Sea, Antarctica. Pol Polar Res 29:17–32
Komárek J, Watanabe M (1990) Morphology and taxonomy of the genus Coleodesmium (Cyanophyceae/Cyanobacteria). In: Watanabe M, Malla SR (eds) Cryptogams of the Himalayas, vol 2, Central and Eastern Nepal, Tsukuba, pp 1–22
Komárek J, Elster J, Komárek O (2008) Diversity of cyanobacterial microflora of the northern part of James Ross Island, NW Weddell Sea, Antarctica. Polar Biol 31:853–865
Kopalová K, Nedbalová L, De Haan M, de Vijver BV (2011) Description of five new species of the diatom genus Luticola (Bacillariophyta, Diadesmidaceae) found in lakes of James Ross Island (Maritime Antarctic Region). Phytotaxa 27:44–60
Matsumoto GI, Friedmann EI, Watanuki K, Ocampo-Friedmann R (1992) Novel long chain anteiso-alkanes and anteiso-alkanoic acids in Antarctic rocks colonized by living and fossil cryptoendolithic microorganisms. J Chromatogr 598:267–276
Nadeau TL, Milbrandt EC, Castenholz RW (2001) Evolutionary relationships of cultivated Antarctic oscillatorians (cyanobacteria). J Phycol 37:650–654
Nübel U, Garcia-Pichel F, Muyzer G (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332
Ohtani S (1986) Epiphytic algae on mosses in the vicinity of Syowa Station, Antarctica. Mem Natl Inst Polar Res 44:209–219
Ohtani S, Kanda H (1987) Epiphytic algae on the moss community of Grimmia lawiana around Syowa Station, Antarctica. Proc NIPR Symp Polar Biol 1:255–264
Pechar L (1987) Use of an acetone: methanol mixture for the extraction and spectrophotometric determination of chlorophyll-a in phytoplankton. Algolog Stud 46:99–117
Prescott GW (1979) A Contribution to a bibliography of Antarctic and Subantarctic Algae. Bibliotheca Phycol 45:312
Řezanka T, Nedbalová L, Elster J, Cajthaml T, Sigler K (2009) Very-long-chain iso- and anteiso-branched fatty acids in N-acylphosphatidylethanolamines from a natural cyanobacterial mat of Calothrix sp. Phytochemistry 70:655–663
Strunecký O, Elster J, Komárek J (2010) Phylogenetic relationships between geographically separate Phormidium cyanobacteria: is there a link between north and south polar regions? Polar Biol 33:1419–1428
Taton A, Grubisic S, Brambilla E, de Wit R, Wilmotte A (2003) Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular approach. Appl Environ Microbiol 69:5157–5169
Taton A, Grubisic S, Balthasart P, Hodgson DA, Laybourn-Parry J, Wilmotte A (2006) Biogeographical distribution and ecological ranges of benthic cyanobacteria in East Antarctic lakes. FEMS Microbiol Ecol 57:272–289
Vincent WF (2000) Cyanobacterial dominance in the polar regions. In: Whitton B, Potts M (eds) Ecology of the Cyanobacteria: their diversity in space and time. Kluwers Academic Press, The Netherlands, pp 321–340
Wille N (1924) Süsswasseralgen von der Deutschen Südpolar-Expedition auf dem Schiff „Gauss“. I. Teil. Süsswasserlagen vom antarktischen Festlande. Deutsche Südpolar-Expedition 1901–1903. Auftr Reichsmin Botanik 8:373–407
Wilmotte A, Van der Auwera G, De Wachter R (1993) Structure of the 16 S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (‘Mastigocladus laminosus HTF’) strain PCC7518, and phylogenetic analysis. FEBS Lett 317:96–100
Wynn-Williams DD (1991) Aerobiology and colonization in Antarctica. In: Hjelmroos M et al (eds) Proc 4th Internat Conference Aerobiol, Stockholm, 1990, vol 30, pp 380–393
Yilmaz M, Philips EJ, Tillett D (2009) Improved methods for the isolation of cyanobacterial dna from environmental samples. J Phycol 45:517–521
Acknowledgments
This study was conducted during the stay of the authors in the Polish Antarctic station Henryk Arctowski in 2004–2005 (headed by Prof. Stanisław Rakusa-Suszczewski and Prof. Adam Barcikowski) and mainly in the Czech station J.G. Mendel (headed by Prof. Pavel Prošek and Prof. Miloš Barták) in 2007 and 2009. We are indebted particularly to Assoc. Prof. Josef Elster for all support of our work, help, and valuable data. The access to the MetaCentrum computing facilities provided under the programme “Projects of Large Infrastructure for Research, Development, and Innovations” LM2010005 funded by the Ministry of Education, Youth, and Sports of the Czech Republic is appreciated/acknowledged. The technical work in laboratories was performed by Jana Šnokhousová and Dana Švehlová. We thank Dr. Keith Edwards for language correction. The work was supported by grants AV0Z60050516, IAA600050704, MSM6007665801, MSM0021620828 and GA CR 206/08/0318.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komárek, J., Nedbalová, L. & Hauer, T. Phylogenetic position and taxonomy of three heterocytous cyanobacteria dominating the littoral of deglaciated lakes, James Ross Island, Antarctica. Polar Biol 35, 759–774 (2012). https://doi.org/10.1007/s00300-011-1123-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1123-x