Abstract
Key message
Cadmium sensitivity in sultr1;1 - sultr1;2 double mutant with limiting sulfate supply is attributed to the decreased glutathione content that affected oxidative defense but not phytochelatins’ synthesis.
Abstract
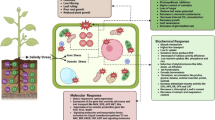
In plants, glutathione (GSH) homeostasis plays pivotal role in cadmium (Cd) detoxification. GSH is synthesized by sulfur (S) assimilation pathway. Many studies have tried to investigate the role of GSH homeostasis on Cd tolerance using mutants; however, most of them have focused on the last few steps of S assimilation. Until now, mutant evidence that explored the relationship between GSH homeostasis on Cd tolerance and S absorption is rare. To further reveal the role of GSH homeostasis on Cd stress, the wild-type and a sultr1;1-sultr1;2 double mutant which had a defect in two distinct high-affinity sulfate transporters were used in this study. Growth parameters, biochemical or zymological indexes and S assimilation-related genes’ expression were compared between the mutant and wild-type Arabidopsis plants. It was found that the mutations of SULTR1;1 and SULTR1;2 did not affect Cd accumulation. Compared to the wild-type, the double mutant was more sensitive to Cd under limited sulfate supply and suffered from stronger oxidative damage. More importantly, under the same condition, lower capacity of S assimilation resulted in decreased GSH content in mutant. Faced to the limited GSH accumulation, mutant seedlings consumed a large majority of GSH in pool for the synthesis of phytochelatins rather than participating in the antioxidative defense. Therefore, homeostasis of GSH, imbalance between antioxidative defense and severe oxidative damage led to hypersensitivity of double mutant to Cd under limited sulfate supply.








Similar content being viewed by others
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali M, Chun H, Kim B, Lee C (2002) Cadmium-induced changes in antioxidant enzyme activities in rice (Oryza sativa L. cv. Dongjin). J Plant Biol 45:134–140
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Astolfi S, Zuchi S, Passera C (2004) Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J Plant Physiol 161:795–802
Azpilicueta CE, Benavides MP, Tomaro ML, Gallego SM (2007) Mechanism of CATA3 induction by cadmium in sunflower leaves. Plant Physiol Biochem 45:589–595
Baker A, Reeves R, Hajar A (2006) Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. and C. Presl (Brassicaceae). New Phytol 127:61–68
Barberon M, Berthomieu P, Clairotte M, Shibagaki N, Davidian JC, Gosti F (2008) Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol 180:608–619
Bashir H, Ahmad J, Bagheri R, Nauman M, Qureshi MI (2013) Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ Exp Bot 94:19–32
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16:1143–1162
Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, Dong XJ, He JX, Pei ZM, Zheng HL (2011) Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot 62:4481–4493
Chen J, Wang WH, Wu FH, You CY, Liu TW, Dong XJ, He JX, Zheng HL (2012a) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Chen J, Wu FH, Liu TW, Chen L, Xiao Q, Dong XJ, He JX, Pei ZM, Zheng HL (2012b) Emissions of nitric oxide from 79 plant species in response to simulated nitrogen deposition. Environ Pollut 160:192–200
Cho UH, Seo NH (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Cho UH, Seo NH (2006) Changes of thiols and oxidative stress in tomato seedlings exposed to cadmium. J Ecol Field Biol 29:61–67
Chu CC, Lee WC, Guo WY, Pan SM, Chen LJ, Li HM, Jinn TL (2005) A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol 139:425–436
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol 3:211–216
Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J 16:73–78
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Di Cagno R, Guidi L, De Gara L, Soldatini GF (2001) Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol 151:627–636
Ebbs S, Uchil S (2008) Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. Photosynthetica 46:49–55
El-Zohri M, Odjegba V, Ma L, Rathinasabapathi B (2015) Sulfate influx transporters in Arabidopsis thaliana are not involved in arsenate uptake but critical for tissue nutrient status and arsenate tolerance. Planta 241:1109–1118
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Frachisse JM, Thomine S, Colcombet J, Guern J, Barbier-Brygoo H (1999) Sulfate is both a substrate and an activator of the voltage-dependent anion channel of Arabidopsis hypocotyl cells. Plant Physiol 121:253–262
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Guan C, Ji J, Jia C, Guan W, Li X, Jin C, Wang G (2015) A GSHS-like gene from Lycium chinense maybe regulated by cadmium-induced endogenous salicylic acid and overexpression of this gene enhances tolerance to cadmium stress in Arabidopsis. Plant Cell Rep 34:871–884
Harada E, Choi YE, Tsuchisaka A, Obata H, Sano H (2001) Transgenic tobacco plants expressing a rice cysteine synthase gene are tolerant to toxic levels of cadmium. J Plant Physiol 158:655–661
Hawkesford MJ (2000) Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J Exp Bot 51:131–138
Hegedüs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Heiss S, Schäfer H, Haag-Kerwer A, Rausch T (1999) Cloning sulfur assimilation genes of Brassica juncea L.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase. Plant Mol Biol 39:847–857
Hodges DM, Forney CF (2000) The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J Exp Bot 51:645–655
Hou W, Chen X, Song G, Wang Q, Chi Chang C (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45:62–69
Howden R, Andersen CR, Goldsbrough PB, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107:1067–1073
Hsu YT, Kao CH (2007) Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil 298:231–241
Hubberten HM, Drozd A, Tran BV, Hesse H, Hoefgen R (2012) Local and systemic regulation of sulfur homeostasis in roots of Arabidopsis thaliana. Plant J 72:625–635
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Koffler BE, Polanschütz L, Zechmann B (2014) Higher sensitivity of pad2-1 and vtc2-1 mutants to cadmium is related to lower subcellular glutathione rather than ascorbate contents. Protoplasma 251:755–769
Kopriva S, Mugford S, Matthewman C, Koprivova A (2009) Plant sulfate assimilation genes: redundancy versus specialization. Plant Cell Rep 28:1769–1780
Lancilli C, Giacomini B, Lucchini G, Davidian JC, Cocucci M, Sacchi GA, Nocito FF (2014) Cadmium exposure and sulfate limitation reveal differences in the transcriptional control of three sulfate transporter (Sultr1; 2) genes in Brassica juncea. BMC Plant Biol 14:132
Lee S, Leustek T (1999) The affect of cadmium on sulfate assimilation enzymes in Brassica juncea. Plant Sci 141:201–207
Li JX, Gao LJ, Zheng L, Chen JH, Wang JT, Wang XR (2014) Determination of monobromobimane-labeled phytochelatins by high-performance liquid chromatography. Anal Lett 47:2148–2155
Lichtenthaler H (1987) Chlorophylls and carotenoids-pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Liu D, An Z, Mao Z, Ma L, Lu Z (2015) Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus STGCS-GS in the presence of Cd, Zn and Cu alone or in combination. PLoS One 10:e0128824
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158
May MJ, Vernoux T, Leaver C, Montagu MV, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49:649–667
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MN (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Momodu M, Anyakora C (2010) Heavy metal contamination of ground water: the surulere case study. Res J Environ Earth Sci 2:39–43
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol 22:867–880
Nocito FF, Pirovano L, Cocucci M, Sacchi GA (2002) Cadmium-induced sulfate uptake in maize roots. Plant Physiol 129:1872–1879
Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian JC, Sacchi GA (2006) Heavy metal stress and sulfate uptake in maize roots. Plant Physiol 141:1138–1148
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Paradiso A, Berardino R, de Pinto MC, Sanita di Toppi L, Storelli MM, Tommasi F, De Gara L (2008) Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69:278–288
Rea PA (2012) Phytochelatin synthase: of a protease a peptide polymerase made. Physiol Plant 145:154–164
Ruiz J, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Ryan KC, Johnson OE, Cabelli DE, Brunold TC, Maroney MJ (2010) Nickel superoxide dismutase: structural and functional roles of Cys2 and Cys6. J Biol Inorg Chem 15:795–807
Saito K (2000) Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol 3:188–195
Salin ML (2007) Toxic oxygen species and protective systems of the chloroplast. Physiol Plantarum 72:681–689
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Semane B, Cuypers A, Smeets K, Van Belleghem F, Horemans N, Schat H, Vangronsveld J (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129:519–528
Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1; 2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29:475–486
Singh P, Tewari R (2003) Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J Environ Biol 24:107–112
Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere A, Vangronsveld J (2005) Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol Biochem 43:437–444
Sneller FEC, van Heerwaarden LM, Koevoets PL, Vooijs R, Schat H, Verkleij JA (2000) Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman’s reagent and monobromobimane. J Agr Food Chem 48:4014–4019
Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377:369–381
Sun X, Lu B, Huang S, Mehta S, Xu L, Yang Z (2007) Coordinated expression of sulfate transporters and its relation with sulfur metabolites in Brassica napus exposed to cadmium. Bot Stud 48:43–54
Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2001) The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J 23:171–182
Talukdar D (2012) An induced glutathione-deficient mutant in grass pea (Lathyrus sativus L.): modifications in plant morphology, alteration in antioxidant activities and increased sensitivity to cadmium. Biorem Biodivers Bioavailab 6:75–86
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Vidmar JJ, Tagmount A, Cathala N, Touraine B, Davidian JCE (2000) Cloning and characterization of a root specific high-affinity sulfate transporter from Arabidopsis thaliana. FEBS Lett 475:65–69
Vögeli-Lange R, Wagner GJ (1996) Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci 114:11–18
Xu J, Yin H, Li X (2009) Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep 28:325–333
Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, Liu X (2012) Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol 196:125–138
Yan K, Chen W, Zhang G, Xu S, Liu Z, He X, Wang L (2010) Elevated CO2 ameliorated oxidative stress induced by elevated O3 in Quercus mongolica. Acta Physiol Plant 32:375–385
Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K (2002) Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J 29:465–473
Yoshimoto N, Inoue E, Watanabe-Takahashi A, Saito K, Takahashi H (2007) Posttranscriptional regulation of high-affinity sulfate transporters in Arabidopsis by sulfur nutrition. Plant Physiol 145:378–388
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zhang ZC, Chen BX, Qiu BS (2010) Phytochelatin synthesis plays a similar role in shoots of the cadmium hyperaccumulator Sedum alfredii as in non-resistant plants. Plant Cell Environ 33:1248–1255
Acknowledgments
We are grateful to Dr. Françoise Gosti from France for kindly providing sultr1;1-sultr1;2 double mutant seeds. This study was financially supported by the Natural Science Foundation of China (NSFC Nos. 30930076, 31260057, 31300505 and 31570586), Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Nanjing Institute of Soil Science, Chinese Academy of Science (Y412201449), China Postdoctoral Science Foundation (2012M521278).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contribution statement
X.L., F.H.W. and H.L.Z. designed the experiments and wrote the manuscript. X.L., F.H.W., J.X.L., J.C., G.H.W., W.H.W., W.J.H., L.J.G., Z.L.W. and J.H.C. conducted experiments. X.L. and M.S. analyzed data. F.H.W. and W.H.W. provided technical assistance. All authors read and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Feher.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
Supplementary Table S1. Lists of primers used in quantitative real-time PCR. Supplementary Table S2. Sulfate uptake or metabolism related genes expression in wild-type and double sultr1;1-sultr1;2 null mutant Arabidopsis seedlings under limited or sufficient sulfate conditions for two weeks. Supplementary Methods: Determination of phytochelatins (PCs). Supplementary Figure S1. The primary root length of ten-day-old wild-type and sultr1;1-sultr1;2 double mutant seedlings grown in 1/2 modified MS medium supplemented with 0, 25, 50, 100, 200, 400 and 1500 μM K2SO4 concentrations. 1/2 standard MS medium was chosen as control. Forty seedlings were used for each treatment. Data are mean ± SE. Supplementary Figure S2. The phenotype of wild-type and double sultr1;1-sultr1;2 null mutant of Arabidopsis seedlings under four treatment conditions for two weeks. (a) LS: 200 μM K2SO4, wild-type; (b) LS+Cd: 200 μM K2SO4 + 20 μM CdCl2, wild-type; (c) SS: 1500 μM K2SO4, wild-type; (d) SS+Cd: 1500 μM K2SO4 + 20 μM CdCl2, wild-type; (e) LS: 200 μM K2SO4 , double sultr1;1-sultr1;2 null mutant; (f) LS+Cd: 200 μM K2SO4 + 20 μM CdCl2, double mutant; (g) SS: 1500 μM K2SO4, double mutant; (h) SS+Cd: 1500 μM K2SO4 + 20 μM CdCl2, double mutant. (DOCX 2048 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Wu, FH., Li, JX. et al. Glutathione homeostasis and Cd tolerance in the Arabidopsis sultr1;1-sultr1;2 double mutant with limiting sulfate supply. Plant Cell Rep 35, 397–413 (2016). https://doi.org/10.1007/s00299-015-1892-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1892-8