Abstract
Key message
An efficient, reproducible, and genotype-independent in planta transformation has been developed for sugarcane using setts as explant.
Abstract
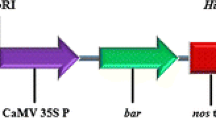
Traditional Agrobacterium-mediated genetic transformation and in vitro regeneration of sugarcane is a complex and time-consuming process. Development of an efficient Agrobacterium-mediated transformation protocol, which can produce a large number of transgenic plants in short duration is advantageous. Hence, in the present investigation, we developed a tissue culture-independent in planta genetic transformation system for sugarcane using setts collected from 6-month-old sugarcane plants. The sugarcane setts (nodal cuttings) were infected with three Agrobacterium tumefaciens strains harbouring pCAMBIA 1301–bar plasmid, and the transformants were selected against BASTA®. Several parameters influencing the in planta transformation such as A. tumefaciens strains, acetosyringone, sonication and exposure to vacuum pressure, have been evaluated. The putatively transformed sugarcane plants were screened by GUS histochemical assay. Sugarcane setts were pricked and sonicated for 6 min and vacuum infiltered for 2 min at 500 mmHg in A. tumefaciens C58C1 suspension containing 100 µM acetosyringone, 0.1 % Silwett L-77 showed the highest transformation efficiency of 29.6 % (with var. Co 62175). The three-stage selection process completely eliminated the chimeric transgenic sugarcane plants. Among the five sugarcane varieties evaluated using the standardized protocol, var. Co 6907 showed the maximum transformation efficiency (32.6 %). The in planta transformation protocol described here is applicable to transfer the economically important genes into different varieties of sugarcane in relatively short time.




Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog medium
- hpt II:
-
Hygromycin phosphotransferase
- gus A:
-
β-Glucuronidase gene
- CaMV 35S:
-
Cauliflower mosaic virus 35S promoter
- 35 S poly A:
-
35S Poly A terminator
- Nos ter:
-
Nopaline synthase terminator
References
Adesoye AI, Togun AO, Machuka J (2010) Transformation of cowpea (Vigna unguiculata L. Walp.) by Agrobacterium infiltration. J Appl Biosci 30:1845–1860
Akbulut M, Yücel M, Öktem HA (2008) Analysis and optimization of DNA delivery into chickpea (Cicer arietinum L.) seedlings by Agrobacterium tumefaciens. Afr J Biotechnol 7(8):1011–1017
Altpeter F, Oraby H (2010) Sugarcane. In: Kempken F, Jung C (eds) Genetic modification of plants. Springer, Berlin, pp 453–472
Arencibia AE, Carmona P, Tellez MT, Chan SM, Yu L, Trujillo Oramas P (1998) An efficient protocol for sugarcane (Saccharum spp.) transformation mediated by Agrobacterium tumefaciens. Transgenic Res 7:213–222
Ashby AM, Watson MD, Loake GJ, Shaw CH (1988) Ti plasmid-specified chemo taxis of Agrobacterium tumefaciens C58C1 towards vir inducing phenolic compounds and soluble factors from monocotyledonous and dicotyledonous plants. J Bacteriol 170:4181–4187
Attia OA, Mohamed AM, Hafez MA, Sadiq AS, Abdallah NA (2005) Establishment of regeneration and transformation systems of F144 sugarcane cultivar. Pak J Biotechnol 2:15–18
Bakshi S, Sadhukhan A, Mishra S, Sahoo L (2011) Improved Agrobacterium-mediated transformation of cowpea via sonication and vacuum infiltration. Plant Cell Rep 30:2281–2292
Chen X, Equi R, Baxter H, Berk K, Han J, Agarwal S, Zale J (2010) A high-throughput transient gene expression system for switchgrass (Panicum virgatum L.) seedlings. Biotechnol Biofuels 3:9
Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980
Chopra R, Apartna Saini R (2012) Use of sonication and vacuum infiltration for Agrobacterium-mediated transformation of an Indian lentil (Lens culinaries Medik.) cultivar. Sci Hort 143:127–134
Chowdhury MKU, Vasil IK (1992) Stably transformed herbicide resistance callus of sugarcane via microprojectile bombardment of cell suspension cultures and electroporation of protoplasts. Plant Cell Rep 11:494–498
Christou P, Ford T, Kofron M (1991) Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio Technol 9:957–962
De Oliveira MLP, Febres VJ, Costa MGC, Moore GA, Otoni WC (2009) High-efficiency Agrobacterium-mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Rep 28:387–395
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA mini preparation: version II. Plant Mol Biol Rep 1:19–21
Eldessoky DS, Ismail RM, Hadi A, Hadi A, Abdallah N (2011) Establishment of regeneration and transformation system of sugarcane cultivar GT54-9 (C9). GM Crops 2(2):126–134
Enríquez-Obregón GA, Vázquez-padrón RI, Prieto-sansonov DL, de la Riva GA, Selman-Housein G (1998) Herbicide resistant sugarcane (Saccharum officinarum L.) plants by Agrobacterium-mediated transformation. Planta 206:20–27
FAOSTAT (2013) Agricultural data. http://faostat.fao.org/site/339/default.aspx
Feldmann KA, Marks MD (1987) Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. Mol Gen Genet 208:1–9
Fromm ME, Morrish F, Armstrong C, Williams R, Thomas J, Klein TM (1990) Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Bio Technol 8:833–838
Goldberg JB, Ohman DE (1984) Cloning and expression in Pseudomonas aeruginosa of a gene involved with the production of alginate. J Bacteriol 158:1115–1121
Hood EE, Helmer GC, Fraley RT, Chilton MD (1986) The hypervirulence of Agrobacterium tumefaciens A281 is encoded in the region of pTiBo542 outside the T-DNA. J Bacteriol 168:1291–1301
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgen Res 2:208–218
Indurker S, Misra HS, Eapen S (2010) Agrobacterium-mediated transformation in chickpea (Cicer arietinum L.) with an insecticidal protein gene: optimization of different factors. Physiol Mol Biol Plant 16(3):273–284
Jaganath B, Subramanyam K, Mayavan S, Karthik S, Elayaraja D, Udayakumar R, Manickavasagam M, Ganapathi A (2014) An efficient in planta transformation of Jatropha curcas (L.) and multiplication of transformed plants through in vivo grafting. Protoplasma 251:591–601
Jefferson RA, Kavanagh TA, Bevan NW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Joersbo M, Brunstedt J (1992) Sonication: a new method for gene transfer to plants. Physiol Plant 85:230–234
Joyce P, Kuwahata M, Turner N, Lakshmanan P (2010) Selection system and co-cultivation medium are important determinants of Agrobacterium-mediated transformation of sugarcane. Plant Cell Rep 29:173–183
Joyce P, Hermann S, O’Connell A, Dinh Q, Shumbe L, Lakshmanan P (2014) Field performance of transgenic sugarcane produced using Agrobacterium and biolistics methods. Plant Biotech J 12:411–424
Khamrit R, Jaisil P, Bunnag S (2012) Callus induction, regeneration and transformation of sugarcane (Saccharum officinarum L.) with chitinase gene using particle bombardment. Afr J Biotechnol 11(24):6612–6618
Lakshmanan P, Geijskes RJ, Wang L, Elliott A, Grof CPL, Berding N, Smith GR (2006) Developmental and hormonal regulation of direct shoot organogenesis and somatic embryogenesis in sugarcane (Saccharum spp. interspecific hybrids) leaf culture. Plant Cell Rep 25:1007–1015
Lam E, Shine J, Silva J, Lawton M, Bonos S, Calvino M, Arrer H, Silva-Filho MC, Glynn N, Helsel Z, Ma J, Richard E, Souza GM, Ming R (2009) Improving sugarcane for biofuel: engineering for an even better feedstock. GCB Bioenergy 1:251–255
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnol NY 9:963–967
Li S, Zhao DG, Wu YJ, Tian X (2009a) A simplified seed transformation method for obtaining transgenic Brassica napus plants. Agric Sci China 8(6):658–663
Li JF, Park E, von Arnim AG, Nebenführ A (2009b) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Method 5:6.A
Lin J, Zhou B, Yang Y, Mei J, Zhao X, Guo X, Huang X, Tang D, Liu X (2009) Piercing and vacuum infiltration of the mature embryo: a simplified method for Agrobacterium-mediated transformation of indica rice. Plant Cell Rep 28:1065–1074
Mamontova EM, Velikov VA, Volokhina IV, Chumakov MI (2010) Agrobacterium-mediated in planta transformation of maize germ cells. Russ J Genet 46:501–504
Manickavasagam M, Ganapathi A, Anbazhagan VR, Sudhakar B, Selvaraj N, Vasudevan A, Kasthurirengan S (2004) Agrobacterium-mediated genetic transformation and development of herbicide resistant sugarcane (Saccharum species hybrids) using axillary buds. Plant Cell Rep 23:134–143
Mayavan S, Subramanyam K, Arun M, Rajesh M, Dev GK, Sivanandhan G, Jaganath B, Manickavasagam M, Selvaraj N, Ganapathi A (2013) Agrobacterium tumefaciens-mediated in planta seed transformation strategy in sugarcane. Plant Cell Rep 32:1557–1574
Mengiste T, Amedeo P, Paszkowski J (1997) High-efficiency transformation of Arabidopsis thaliana with a selectable marker gene regulated by the T-DNA 19 promoter. Plant J 12:945–948
Ming R, Moore PH, Wu KK, D’Hont A, Glaszmann JC, Tew TL (2006) Sugarcane improvement through breeding and biotechnology. Plant Breed Rev 27:15–118
Mordocco AM, Brumbley JA, Lakshmanan P (2009) Development of a temporary immersion system (RITA®) for mass production of sugarcane (Saccharum spp. interspecific hybrids). In Vitro Cell Dev Biol Plant 45:450–457
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naseri G, Sohani MM, Pourmassalehgou A, Allahi S (2012) In planta transformation of rice (Oryza sativa) using thaumatin-like protein gene for enhancing resistance to sheath blight. Afr J Biotechnol 11(31):7885–7893
Pawlicki N, Sangwan RS, Sangwan-Norreel BS (1992) Factors influencing the Agrobacterium tumefaciens-mediated transformation of carrot (Daucus carota L.). Plant Cell Tissue Org Cult 31:129–139
Pérez-Jiménez M, Besnard G, Dorado G, Hernandez P (2013) Varietal tracing of virgin olive oils based on plastid DNA variation profiling. PLoS One 8(8):1
Qing CM, Fan L, Lei Y, Bouchez D, Tourneur C, Yan L, Robaglia C (2000) Transformation of Pakchoi (Brassica rapa L. ssp. chinensis) by Agrobacterium infiltration. Mol Breed 6:67–72
Sambrook J, Fritch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring, Harbor
Seol E, Jung Y, Lee J, Cho C, Kim T, Rhee Y, Lee S (2008) In planta transformation of Notocactus scopa cv. Soonjung by Agrobacterium tumefaciens. Plant Cell Rep 27:1197–1206
Solís JIF, Mlejnek P, Studená K, Procházka S (2003) Application of sonication-assisted Agrobacterium-mediated transformation in Chenopodium rubrum L. Plant Soil Environ 49(6):255–260
Subramaniam S, Samian R, Midrarullah RathinamX (2009) Preliminary factors influencing transient expression of gus A in Dendrobium savin white protocorm-like bodies (PLBs) using Agrobacterium-mediated transformation system. WASJ 7:1295–1307
Subramanyam K, Subramanyam K, Sailaja KV, Srinivasulu M, Lakshmidevi K (2011) Highly efficient Agrobacterium-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. Plant Cell Rep 30:425–436
Subramanyam K, Rajesh M, Jaganath B, Vasuki A, Theboral J, Elayaraja D, Karthik S, Manickavasagam M, Ganapathi A (2013) Assessment of factors influencing the Agrobacterium-mediated in planta transformation of brinjal (Solanum melongena L.). Appl Biochem Biotech 171:450–468
Supartana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M (2005) Development of simple and efficient in planta transformation method for rice (Oryza zativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100:391–397
Supartana P, Shimizu T, Nogawa M, Shioiri H, Nakajima T, Haramoto N, Nozue M, Kojima M (2006) Development of simple and efficient in planta transformation method for wheat (Triticum aestivum L.) using Agrobacterium tumefaciens. J Biosci Bioeng 102:162–170
Tague BW, Mantis J (2006) In planta Agrobacterium-mediated transformation by vacuum infiltration. Method Mol Biol 323:215–223
Taparia Y, Gallo M, Altpeter F (2012) Comparision of direct and indirect embryogenesis protocols, biolistic gene transfer and selection parameters for efficient genetic transformation of sugarcane. Plant Cell Tiss Organ Cult 111:131–141
Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA, Shin H, Chiou TJ, Katagi H, Dewbre GR, Weigel D, Harrison MJ (2000) Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. Plant J 22:531–541
Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, Schell J (1974) Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature 252:169–170
Vasil V, Castillo AM, Fromm EM, Vasil IK (1992) Herbicide resistant transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Bio Technol 10:667–674
Wang Q, Xing S, Pan Q, Yuan F, Zhao J, Tian Y, Chen Y, Wang G, Tang K (2012) Development of efficient catharanthus roseus regeneration and transformation system using Agrobacterium tumefaciens and hypocotyls as explants. BMC Biotech 12:34
Wilson K, Long D, Swinburne J, Coupland G (1996) A Dissociation insertion causes a semidominant mutation that increases expression of TINY, an Arabidopsis gene related to APETALA2. Plant Cell 8:659–671
Zale JM, Agarwal S, Loar S, Steber CM (2009) Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens. Plant Cell Rep 28:903–913
Zhangsun DT, Luo SL, Chen RK, Tang KX (2007) Improved Agrobacterium-mediated genetic transformation of GNA transgenic sugarcane. Biologia 62(4):386–393
Acknowledgments
The authors are grateful to University Grants Commission (UGC), Government of India, for the financial support [No.F.31-239/2005 (SR)] to carry out the present work. The corresponding author is thankful to University Grants Commission (UGC), Govt. of. India for providing Fellowship under UGC–BSR scheme. All the authors are thankful to Prof. A.S Rao, Department of Biotechnology & Genetic Engineering, Bharathidasan University, Tiruchirappalli for his valuable suggestions in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. Lakshmanan.
Subramanian Mayavan and Kondeti Subramanyam contributed equally.
Rights and permissions
About this article
Cite this article
Mayavan, S., Subramanyam, K., Jaganath, B. et al. Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep 34, 1835–1848 (2015). https://doi.org/10.1007/s00299-015-1831-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1831-8