Abstract
Key message
Arabidopsis Ca 2+ -ATPase ACA8 plays a role in sucrose signalling during early seedling development by integrating developmental signals with carbon source availability.
Abstract
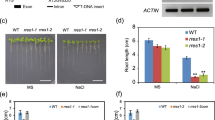
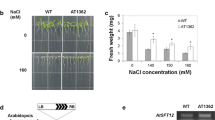
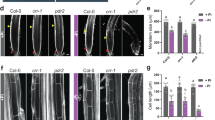
Calcium (Ca2+) is an essential signal transduction element in eukaryotic organisms. Changes in the levels of intracellular Ca2+ affect multiple developmental processes in plants, including cell division, polar growth, and organogenesis. Here, we report that the plasma-membrane-localised Arabidopsis Ca2+-ATPase ACA8 plays a role in sucrose signalling during early seedling development. Disruption of the ACA8 gene elevated the expression of genes that encode transporters for Ca2+ efflux. The seedlings that carried a T-DNA insertion mutation in ACA8 experienced water stress during early development. This response was unrelated to inadequate osmoregulatory responses and was most likely caused by disruption of cell membrane integrity and severe ion leakage. In addition, aca8-1 seedlings displayed a significant decline in photosynthetic performance and arrested root growth after removal of sucrose from the growth medium. The two phenomena resulted from impaired photosynthesis, reduced cell proliferation in the root meristem and the sucrose control of cell-cycle events. All of the stress-response phenotypes were rescued when expression of ACA8 was restored in aca8-1 mutant. Taken together, our results indicate that ACA8-mediated Ca2+ signalling contributes to modulate early seedling development and coordinates root development with nutrient availability.








Similar content being viewed by others
References
Ainsworth EA, Bush DR (2011) Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol 155:64–69
Batistič O, Kudla J (2010) Calcium: not just another ion. Cell biology of metals and nutrients. Springer, Berlin, pp 17–54
Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berger S (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225:1–12
Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S (2011) Calcium efflux systems in stress signaling and adaptation in plants. Front Plant Sci 2:85
Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, Harper JF (2010) Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol 154:1158–1171
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97:3718–3723
de Jager SM, Maughan S, Dewitte W, Scofield S, Murray JA (2005) The developmental context of cell-cycle control in plants. Semin Cell Dev Biol 16:385–396
Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61:593–620
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Eveland AL, Jackson DP (2012) Sugars, signalling, and plant development. J Exp Bot 63:3367–3377
Frei dit Frey N, Mbengue M, Kwaaitaal M, Nitsch L, Altenbach D, Haweker H, Lozano-Duran R, Njo MF, Beeckman T, Huettel B, Borst JW, Panstruga R, Robatzek S (2012) Plasma membrane calcium ATPases are important components of receptor-mediated signaling in plant immune responses and development. Plant Physiol 159:798–809
Geiger D (2011) Plant sucrose transporters form a biophysical point of view. Mol Plant 4:395–406
George L, Romanowsky SM, Harper JF, Sharrock RA (2008) The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol 146:716–728
Giacometti S, Marrano CA, Bonza MC, Luoni L, Limonta M, De Michelis MI (2012) Phosphorylation of serine residues in the N-terminus modulates the activity of ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana. J Exp Bot 63:1215–1224
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155
Hirschi KD (1999) Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11:2113–2122
Hohl M, Schopfer P (1991) Water relations of growing Maize coleoptiles comparison between Mannitol and Polyethylene glycol 6000 as external osmotica for adjusting turgor pressure. Plant Physiol 95:716–722
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant cell 22:541–563
McAinsh MR, Pittman JK (2009) Shaping the calcium signature. New Phytol 181:275–294
Menges M, de Jager SM, Gruissem W, Murray JA (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41:546–566
Minocha R, Martinez G, Lyons B, Long S (2009) Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can J For Res 39:849–861
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3:973–996
Pego JV, Kortstee AJ, Huijser C, Smeekens SC (2000) Photosynthesis, sugars and the regulation of gene expression. J Exp Bot 51:407–416
Riou-Khamlichi C, Menges M, Healy JS, Murray JA (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 20:4513–4521
Sanchez I, Dynlacht BD (2005) New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol 16:311–321
Schiøtt M, Romanowsky SM, Bækgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101:9502–9507
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37:501–506
Skylar A, Hong F, Chory J, Weigel D, Wu X (2010) STIMPY mediates cytokinin signaling during shoot meristem establishment in Arabidopsis seedlings. Development 137:541–549
Skylar A, Sung F, Hong F, Chory J, Wu X (2011) Metabolic sugar signal promotes Arabidopsis meristematic proliferation via G2. Dev Biol 351:82–89
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279
Sze H, Liang F, Hwang I, Curran AC, Harper JF (2000) Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Ann Rev Plant Biol 51:433–462
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616
Van Leene J, Hollunder J, Eeckhout D, Persiau G, Van De Slijke E, Stals H, Van Isterdael G, Verkest A, Neirynck S, Buffel Y, De Bodt S, Maere S, Laukens K, Pharazyn A, Ferreira PC, Eloy N, Renne C, Meyer C, Faure JD, Steinbrenner J, Beynon J, Larkin JC, Van de Peer Y, Hilson P, Kuiper M, De Veylder L, Van Onckelen H, Inze D, Witters E, De Jaeger G (2010) Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol 6:397
Van’t Hof J (1966) Experimental control of DNA synthesizing and dividing cells in excised root tips of Pisum. Am J Bot 53:970–976
Van’t Hof J, Rost T (1972) Cell proliferation in complex tissues: the control of the mitotic cycle of cell populations in the cultured root meristem of sunflower (Helianthus). Am J Bot 53:970–976
Wahl V, Brand LH, Guo YL, Schmid M (2010) The FANTASTIC FOUR proteins influence shoot meristem size in Arabidopsis thaliana. BMC Plant Biol 10:285
Wu X, Dabi T, Weigel D (2005) Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr Biol 15:436–440
Zhang L, Zhao G, Xia C, Jia J, Liu X, Kong X (2012) A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J Exp Bot 63:5873–5885
Ziv M (1990) Vitrification: morphological and physiological disorders of in vitro plants. Micropropagation. Springer, Berlin, pp 45–69
Acknowledgments
The authors thank Dr. Yanxia Jia for allowing the use of confocal laser scanning microscope, Ruoxi Fan for assistance on gene expression analysis, and Xiangshi Qin for plant growth. The research was supported by grants from the National Natural Science Foundation of China (NSFC 31300251), Kunming Institute of Botany (KSCX2-EW-J-24), Germplasm Bank of Wild Species, and CAS Innovation Program of Kunming Institute (540806321211), and 100-Talents Program of CAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Zhang, X., Wang, R. et al. The plasma membrane-localised Ca2+-ATPase ACA8 plays a role in sucrose signalling involved in early seedling development in Arabidopsis . Plant Cell Rep 33, 755–766 (2014). https://doi.org/10.1007/s00299-014-1590-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1590-y