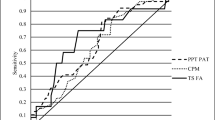
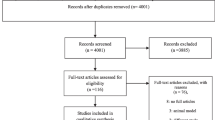
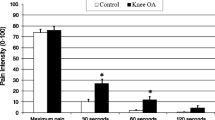
Abstract
There are no standardized bedside assessments for subtyping patients with osteoarthritis (OA) based on pain mechanisms. Thus, we developed a bedside sensory testing kit (BSTK) to classify OA patients based on sensory profiles potentially indicative of pain mechanism. After usability and informal reliability testing (n = 22), the kit was tested in a formal reliability study (n = 20). Patients completed questionnaires and sensory testing: pressure algometry to detect hyperalgesia; repeat algometry after heterotopic noxious conditioning stimulation to measure diffuse noxious inhibitory control (DNIC); light touch using Von Frey filaments; and cold allodynia using a brass rod. The procedure was brief and well tolerated. Algometry and filament testing were highly reliable [intra-class correlation coefficients (ICCs) 0.71–0.91]; DNIC was acceptably reliable (ICCs 0.53–0.91); brass rod reliability was inconclusive. Patients were classified empirically into four groups: “All abnormal findings” (primary and secondary hyperalgesia and dysfunctional DNIC); “all normal findings”; and two intermediate groups. The “all abnormal findings” group had more neuropathic pain symptoms, and lower WOMAC total, stiffness, and activity scores than the “all normal findings” group. Simple BSTK procedures, consolidated in a kit, reliably classified OA patients into subgroups based on sensory profile, suggesting that OA patients differ in underlying pain mechanisms. Further research is needed to confirm these subgroups and determine their validity in predicting response to treatment.


Similar content being viewed by others
Abbreviations
- APAP:
-
Acetaminophen
- BSTK:
-
Bedside sensory testing kit
- DNIC:
-
Diffuse noxious inhibitory control
- HA:
-
Hyperalgesia/allodynia
- HNCS:
-
Heterotopic noxious conditioning stimulus
- ICC:
-
Intra-class correlation coefficient
- LTT:
-
Light touch threshold
- NRS:
-
Numerical Rating Scale
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- PPT:
-
Pressure pain threshold
- PQAS:
-
Pain Quality Assessment Scale
- QDS:
-
Questionnaire Development Systems
- QST:
-
Quantitative sensory testing
- WOMAC:
-
Western Ontario McMaster Osteoarthritis Questionnaire
References
Melton L (2003) Osteoarthritis pain goes central. Lancet Neurol 2:524
Dieppe PA, Lohmander LS (2005) Pathogenesis and management of pain in osteoarthritis. Lancet 365:965–973
Bedson J, Croft PR (2008) The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 9:116
Kosek E, Ordeberg G (2000) Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 4:229–238
Woolf CJ, American College of Physicians, American Physiological Society (2004) Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 140:441–451
Graven-Nielsen T, Arendt-Nielsen L (2002) Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep 4:313–321
Dray A, Read SJ (2007) Future targets to control osteoarthritis pain. Arthritis Res Ther 9:212
Wong HY (1993) Neural mechanisms of joint pain. Ann Acad Med Singap 22:646–650
Schaible HG, Ebersberger A, Von Banchet GS (2002) Mechanisms of pain in arthritis. Ann N Y Acad Sci 966:343–354
Farrell M, Gibson S, McMeeken J, Helme R (2000) Pain and hyperalgesia in osteoarthritis of the hands. J Rheumatol 27:441–447
Moss P, Sluka K, Wright A (2007) The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther 12:109–118
Kidd BL, Langford RM, Wodehouse T (2007) Arthritis and pain. Current approaches in the treatment of arthritic pain. Arthritis Res Ther 9:214
Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L (2001) Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain 93:107–114
Farrell MJ, Gibson SJ, McMeeken JM, Helme RD (2000) Increased movement pain in osteoarthritis of the hands is associated with a beta-mediated cutaneous mechanical sensitivity. J Pain 1:229–242
Bradley LA (2004) Recent approaches to understanding osteoarthritis pain. J Rheumatol Suppl 70:54–60
Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T (2003) Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain 4:203–211
Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M (2007) The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg 105:815–821
Kosek E, Ordeberg G (2000) Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 88:69–78
Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS (2006) The Pain Quality Assessment Scale: assessment of pain quality in carpal tunnel syndrome. J Pain 7:823–832
Jensen K, Andersen HO, Olesen J, Lindblom U (1986) Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain 25:313–323
Bailey KD (1984) A three-level measurement model. Qual Quant 18:225–245
Pud D, Granovsky Y, Yarnitsky D (2009) The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144:16–19
Lewis JR (1994) Sample sizes for usability studies: additional considerations. Hum Factors 36:368–378
Donner A, Eliasziw M (1987) Sample size requirements for reliability studies. Stat Med 6:441–448
Walter SD, Eliasziw M, Donner A (1998) Stat Med 17:101–110
Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH (1993) Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 70:200–212
Kim JH, Greenspan JD, Coghill RC, Ohara S, Lenz FA (2007) Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci 27:4995–5004
Edwards RR, Haythomthwaite JA, Tella P, Max MB, Raja S (2006) Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology 104:1243–1248
Ardendt-Nielsen L, Yarnitsky D (2009) Experimental and clinical applications of quantitative sensory testing applied to skin, muscles, and viscera. J Pain 10:556–572
Eisenberg E, Midbari A, Haddad M, Pud D (2010) Predicting the analgesic effect to oxycodone by ‘static’ and ‘dynamic’ quantitative sensory testing in healthy patients. Pain 151:104–109
Roth SH (1988) Pharmacologic approaches to musculoskeletal disorders. Clin Geriatr Med 4:441–461
Katz WA (1996) Approach to the management of nonmalignant pain. Am J Med 101:54S–63S
Acknowledgments
This study was funded by a Grant from Merck Research Laboratories. The authors thank Florence Paillard for her assistance in drafting and revising the manuscript.
Conflict of interest
Support for this study was provided by Merck. Eric Osgood has no competing interests to disclose. Thomas Eaton has no competing interests to disclose. Jeremiah Trudeau has no competing interests to disclose. Mark P. Jensen has served as a consultant to Endo Pharmaceuticals, Inc., and has received consulting fees from RTI Health Solutions, Covidien, Bristol-Myers Squibb, Schwartz Biosciences, Analgesic Research, Depomed, Eli Lilly, Pfizer, Merck, Medtronic, and Smith & Nephew within the past 36 months. Arnold Gammaitoni at the time the study was conducted was an employee at Merck. Lee Simon is a principal at SDG consulting, with many clients throughout the medical device and pharmaceutical industries. Nathaniel Katz is the CEO of Analgesic Solutions, a clinical research and consulting firm with many clients throughout the medical device and pharmaceutical industries.
Author information
Authors and Affiliations
Corresponding author
Additional information
E.O., J.T., and T.E. are no longer working at Analgesic Solutions.
Rights and permissions
About this article
Cite this article
Osgood, E., Trudeau, J.J., Eaton, T.A. et al. Development of a bedside pain assessment kit for the classification of patients with osteoarthritis. Rheumatol Int 35, 1005–1013 (2015). https://doi.org/10.1007/s00296-014-3191-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3191-z