Abstract
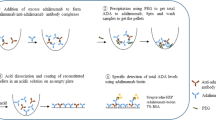
Patients treated with adalimumab (ADL) can induce anti-ADL antibodies (AAA) formation that is associated with low drug levels and clinical non-response. But, in the majority of the assays, the measurement of AAA is hampered by the presence of the drug itself. In support of immunogenicity assessment in clinical samples with subtherapeutic ADL levels, we proved acid pre-treatment for AAA detection with the Promonitor-enzyme-linked immunosorbent assay (ELISA). Were measured AAA after acidification in 32 serum samples with a subtherapeutic ADL trough level. ADL and AAA concentrations were measured by ELISA (Promonitor). The impact of drug concentration on AAA recovery (with or without acidification) was also evaluated by mixing known amounts of ADL (0.25, 0.5 and 1 mg/L) and AAA (100, 200, 300 and 400 AU/mL) from clinical samples in pooled serum. The drug significantly inhibited the detection of AAA in untreated samples. And progressively higher levels of ADL cause increasing inhibition of signal. Acid pre-treatment carried a significant increase in assay response, particularly at lower free ADL concentrations. AAA were detected in the 53 % of the samples after acid dissociation. In seven patients, the positive AAA after dissociation was detected in the first monitoring of ADL and five patients were positive 3 months later for AAA with the standard assay. Monitoring AAA using acid dissociation in patients with subtherapeutic circulating level of ADL could detect precocious problems of bioavailability, assess the immunogenicity of ADL and may be used to optimise dose regimens, thereby preventing prolonged use of inadequate therapy and guide change of treatment.



Similar content being viewed by others
References
Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT et al (2011) Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J 13:99–110
Mok CC, van der Kleij D, Wolbink GJ (2013) Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases. Clin Rheumatol 32:1429–1435
Pascual-Salcedo D, Plasencia C, Ramiro S, Nuño L, Bonilla G, Nagore D et al (2011) Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology 50:1445–1452
Krieckaert CLM, Bartelds GM, Lems WF, Wolbink GJ (2010) The effect of immunomodulators on the immunogenicity of TNF-blocking therapeutic monoclonal antibodies: a review. Arthritis Res Ther 12:217–222
Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M et al (2004) Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods 2004(289):1–16
Koren E, Smith HW, Shores E, Shankar G, Finco-Kent D, Rup B et al (2008) Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods 333:1–9
Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL et al (2009) Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 68:1739–1745
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L et al (2007) Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis 66:921–926
Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW et al (2011) Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305:1460–1468
de Vries MK, Brouwer E, van der Horst-Bruinsma IE, Spoorenberg A, van Denderen JC, Jamnitski A et al (2009) Decreased clinical response to adalimumab in ankylosing spondylitis is associated with antibody formation. Ann Rheum Dis 68:1787–1788
Lecluse LL, Driessen RJ, Spuls PI, de Jong EM, Stapel SO, van Doorn MB et al (2010) Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol 146:127–132
Jamnitski A, Bartelds GM, Nurmohamed MT, van Schouwenburg PA, van Schaardenburg D, Stapel SO et al (2011) The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis 70:284–288
Wolbink GJ, Aarden LA, Dijkmans BA (2009) Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol 21:211–215
Hart MH, de Vrieze H, Wouters D, Wolbink GJ, Killestein J, de Groot ER et al (2011) Differential effect of drug interference in immunogenicity assays. J Immunol Methods 372:196–203
van Schouwenburg PA, Bartelds GM, Hart MH, Aarden L, Wolbink GJ, Wouters D (2010) A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J Immunol Methods 362:82–88
van Schouwenburg PA, van de Stadt LA, de Jong RN, van Buren EEL, Kruithof S, de Groot E et al (2013) Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann Rheum Dis 72:104–109
van Schouwenburg PA, Krieckaert CL, Rispens T, Aarden L, Wolbink GJ, Wouters D (2013) Long-term measurement of anti-adalimumab using pH-shift-anti-idiotype antigen binding test shows predictive value and transient antibody formation. Ann Rheum Dis 72:1680–1686
Bourdage JS, Cook CA, Farrington DL, Chain JS, Konrad RJ (2007) An affinity capture elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. J Immunol Methods 327:10–17
Lofgren JA, Wala I, Koren E, Swanson SW, Jing S (2006) Detection of neutralizing anti-therapeutic protein antibodies in serum or plasma samples containing high levels of the therapeutic protein. J Immunol Methods 308:101–108
Patton A, Mullenix MC, Swanson SJ, Koren E (2005) An acid dissociation bridging ELISA for detection of antibodies directed against therapeutic proteins in the presence of antigen. J Immunol Methods 304:189–195
Schmidt E, Hennig K, Mengede C, Zillikens D, Kromminga A (2009) Immunogenicity of rituximab in patients with severe pemphigus. Clin Immunol 132:334–341
Sickert D, Kroeger K, Zickler C, Chokote E, Winkler B, Grenet JM et al (2008) Improvement of drug tolerance in immunogenicity testing by acid treatment on Biacore. J Immunol Methods 334:29–36
Shankar G, Shores E, Wagner C, Mire-Sluis AR (2006) Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol 24:274–280
Mikulskis A, Yeung D, Subramanyam M, Amaravadi L (2011) Solution ELISA as a platform of choice for development of robust, drug tolerant immunogenicity assays in support of drug development. J Immunol Methods 365:38–49
Butterfield AM, Chain JS, Ackermann BL, Konrad RJ (2010) Comparison of assay formats for drug-tolerant immunogenicity testing. Bioanalysis 2:1961–1969
Smith HW, Butterfield A, Sun D (2007) Detection of antibodies against therapeutic proteins in the presence of residual therapeutic protein using a solid-phase extraction with acid dissociation (SPEAD) sample treatment prior to ELISA. Regul Toxicol Pharmacol 49:230–237
Zhong ZD, Dinnogen S, Hokom M, Ray C, Weinreich D, Swanson SJ et al (2010) Identification and inhibition of drug target interference in immunogenicity assays. J Immunol Methods 355:21–28
Quinn TC, Kline R, Moss MW, Livingston RA, Hutton N (1993) Acid dissociation of immune complexes improves diagnostic utility of p24 antigen detection in perinatally acquired human immunodeficiency virus infection. J Infect Dis 167:1193–1196
Jayarao KS, Faulk WP, Karam JH, Grodsky GM, Forsham PH (1973) Measurement of immune complexes in insulin-treated diabetics. J Immunol Methods 3:337–345
Roch AM, Delcros JG, Ripoll JP, Thomas V, Richard J, Quash G (1990) A novel covalent enzyme-linked immunoassay (CELIA) for simultaneously measuring free and immune complex-bound antibodies of defined specificity: I. Application to naturally occurring antipolyamine antibodies in human sera. J Immunol Methods 133:1–11
Fineberg NS, Fineberg ES, Anderson JH, Birkett MA, Gibson RG, Hufferd S (1996) Immunologic effects of insulin lispro [Lys (B28), Pro (B29) human insulin] in IDDM and NIDDM patients previously treated with insulin. Diabetes 45:1750–1754
Dixon K (1974) Measurement of antibodies to insulin in serum. Clin Chem 20:1275–1281
Llinares Tello F, Rosas Gómez de Salazar J, Senabre Gallego JM, Santos Soler G, Santos Ramírez C, Salas Heredia E et al (2012) Analytical and clinical evaluation of a new immunoassay for therapeutic drug monitoring of infliximab and adalimumab. Clin Chem Lab Med 50:1845–1847
Llinares Tello F, Rosas J, de la Torre I, Valor L, Barber X, Senabre JM et al (2014) Comparative study of both versions of an immunoassay commercialized for therapeutic drug monitoring of adalimumab in rheumatoid arthritis. Reumatol Clín 10:105–108
Ruiz-Argüello B, Ruiz del Agua A, Torres N, Monasterio A, Martínez A, Nagore D (2013) Comparison study of two commercially available methods for the determination of infliximab, adalimumab, etanercept and anti-drug antibody levels. Clin Chem Lab Med 51:e287–e289
Rosas J, Llinares F, de la Torre I, Valor L, Barber X, Santos-Ramírez C et al (2013) Clinical usefulness of serum level of adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 72(Suppl3):333
Wang Y, Fang L, Zhou L, Wang J, Ahn H (2012) A Survey of applications of biological products for drug interference of immunogenicity assays. Pharm Res 29:3384–3392
Gorovits B (2009) Antidrug antibody assay validation: industry survey results. AAPS J 11:133–138
den Broeder AA, van der Maas A, van den Bemt BJF (2010) Dose de-escalation strategies and role of therapeutic drug monitoring of biologics in RA. Rheumatology 49:1801–1803
Bendtzen K (2012) Anti-TNF-α biotherapies: perspectives for evidence-based personalized medicine. Immunotherapy 4:1167–1179
Garcês S, Demengeot J, Benito-García E (2013) The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 72:1947–1955
Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C (2013) Antidrug antibodies to tumour necrosis factor-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 72:165–178
Chen DY, Chen YM, Tsai WC, Tseng JC, Chen YH, Hsieh CW et al. (2014) Ann Rheum Dis. doi:10.1136/annrheumdis-2013-203893
Conflict of interest
The authors have not any conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Group AIRE-MB Association for the Investigation in Rheumatology of the Marina Baixa (AIRE-MB): José Rosas Gómez de Salazar, Esteban Salas Heredia, José Miguel Senabre-Gallego, Gregorio Santos-Soler, Catalina Cano Pérez, Ana Pons Bas, Marisa Lorente Betoret (Rheumatology Department, Marina Baixa Hospital), Francisca Llinares-Tello, Juan Molina García (Laboratory Department, Marina Baixa Hospital); Carlos Santos-Ramírez (Rheumatology Department, Marina Salud Hospital, Denia), Xavier Barber Vallés (CIO-Miguel Hernández University, Elche), Mabel Sánchez Barrioluengo (INGENIO [SIC-UPV], Valencia Polytechnic University).
Rights and permissions
About this article
Cite this article
Llinares-Tello, F., Rosas-Gómez de Salazar, J., Senabre-Gallego, J.M. et al. Practical application of acid dissociation in monitoring patients treated with adalimumab. Rheumatol Int 34, 1701–1708 (2014). https://doi.org/10.1007/s00296-014-3032-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3032-0