Abstract
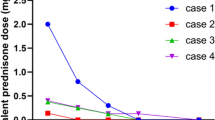
Systemic onset juvenile idiopathic arthritis (SoJIA) is a rare inflammatory disorder. It can result in disease and treatment-related disability. SoJIA is characterized by remitting fevers, evanescent rash, generalized lymphadenopathy, hepatomegaly/splenomegaly, and/or serositis. Non-responsiveness to standard therapy with corticosteroids and disease modifying antirheumatic drugs is not uncommon. IL-1β has been shown to be a main contributor to the pathogenesis of SoJIA. Anakinra, a recombinant IL-1β receptor antagonist, was shown to be effective in small cohorts of therapy-resistant adult and pediatric Still’s patients. In order to assess the efficacy and safety of first-line anakinra treatment in SoJIA, we reviewed the charts of all SoJIA patients in our institution from 2005 to 2010, searching for first-line anakinra-treated patients. We report the clinical and laboratory course of four SoJIA patients. The mean follow-up was 13.5 (range: 2–50) months. Anakinra was started at doses from 1.5 to 4 mg/kg for a median duration of 3 (range: 3–18) months. Two patients responded to anakinra mono-therapy; two cases required corticosteroids. Normalized body temperatures and the absence of evanescent rashes were achieved after a median of 4 (range: 2–10) days. We did not see treatment-related adverse reactions other than local injection site inflammation. This is the first single-center series, reporting anakinra as first-line treatment in SoJIA. We show rapid efficacy of anakinra in early SoJIA with reduced treatment-related side effects. A subset of patients remains corticosteroid dependent. Further studies are warranted to follow larger cohorts and to assess long-term safety.

Similar content being viewed by others
References
Möller JC, Paul D, Ganser G, Range U, Gahr M, Kelsch R, Rösen-Wolff A, Hedrich CM (2010) IL10 promoter polymorphisms are associated with systemic onset juvenile idiopathic arthritis (SoJIA). Clin Exp Rheumatol 28(6):912–918
Youm JY, Woo JH, Kim TH, Bae SC, Yoo DH (2007) Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms in Korean patients with adult-onset Still’s disease. Scand J Rheumatol 36(5):390–393
De Benedetti F, Martini A (1998) Is systemic juvenile rheumatoid arthritis an interleukin 6 mediated disease? J Rheumatol 25(2):203–207
De Benedetti F, Ravelli A, Martini A (1997) Cytokines in juvenile rheumatoid arthritis. Curr Opin Rheumatol 9(5):428–433
Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J (2005) Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 201(9):1479–1486
Hedrich CM, Fiebig B, Sallmann S, Bruck N, Hahn G, Roesler J, Roesen-Wolff A, Heubner G, Gahr M (2008) Good response to IL-1beta blockade by anakinra in a 23-year-old CINCA/NOMID patient without mutations in the CIAS1 gene. Cytokine profiles and functional studies. Scand J Rheumatol 37(5):385–389
Naumann L, Feist E, Natusch A, Langen S, Krause A, Buttgereit F, Burmester GR (2010) IL1-receptor antagonist anakinra provides long-lasting efficacy in the treatment of refractory adult-onset Still’s disease. Ann Rheum Dis 69(2):466–467
Ohlsson V, Baildam E, Foster H, Jandial S, Pain C, Strike H, Ramanan AV (2008) Anakinra treatment for systemic onset juvenile idiopathic arthritis (SOJIA). Rheumatology (Oxford) 47(4):555–556 (Epub 2008 Mar 5)
Gattorno M, Piccini A, Lasigliè D, Tassi S, Brisca G, Carta S, Delfino L, Ferlito F, Pelagatti MA, Caroli F, Buoncompagni A, Viola S, Loy A, Sironi M, Vecchi A, Ravelli A, Martini A, Rubartelli A (2008) The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthr Rheum 58(5):1505–1515
Lequerré T, Quartier P, Rosellini D, Alaoui F, De Bandt M, Mejjad O, Kone-Paut I, Michel M, Dernis E, Khellaf M, Limal N, -Deslandre C, Fautrel B, Le Loët X, Sibilia J (2008) Société Francophone pour la Rhumatologie et les Maladies Inflammatoires en Pédiatrie (SOFREMIP); Club Rhumatismes et Inflammation (CRI). Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset still disease: preliminary experience in France. Ann Rheum Dis 67(3):302–308
Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, Cortis E, Pardeo M, Miettunen PM, Janow G, Birmingham J, Eggebeen A, Janssen E, Shulman AI, Son MB, Hong S, Jones K, Ilowite NT, Cron RQ, Higgins GC (2011) Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthr Rheum 63(2):545–555
Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S, LeBideau M, Bader-Meunier B, Mouy R, Debré M, Landais P, Prieur AM (2003) Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthr Rheum 48(4):1093–1101
Beresford MW, Baildam EM (2009) New advances in the management of juvenile idiopathic arthritis–2: the era of biologicals. Arch Dis Child Educ Pract Ed 94(5):151–156
Durand M, Troyanov Y, Laflamme P, Gregoire G (2010) Macrophage activation syndrome treated with anakinra. J Rheumatol 37(4):879–880
Bruck N, Suttorp M, Kabus M, Heubner G, Gahr M, Pessler F (2010) Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol 17:23–27
Maakaroun NR, Moanna A, Jacob JT, Albrecht H (2010) Viral infections associated with haemophagocytic syndrome. Rev Med Virol 20(2):93–105
Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL (2006) Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 355(6):581–592
Frenkel J, Wulffraat NM, Kuis W (2004) Anakinra in mutation negative NOMID/CINCA syndrome. Arthr Rheum 50:3738–3739
Frosch M, Roth J (2007) Systemische Verlaufsform (Morbus Still). In: Wagner N, Dannecker G (eds) Pädiatrische Rheumatologie, vol 1. Springer, Heidelberg, pp 181–193
Frosch M, Metze D, Foell D, Vogl T, Sorg C, Sunderkötter C, Roth J (2005) Early activation of cutaneous vessels and epithelial cells is characteristic of acute systemic onset juvenile idiopathic arthritis. Exp Dermatol 14(4):259–265
Foell D, Wulffraat N, Wedderburn LR, Wittkowski H, Frosch M, Gerss J, Stanevicha V, Mihaylova D, Ferriani V, Tsakalidou FK, Foeldvari I, Cuttica R, Gonzalez B, Ravelli A, Khubchandani R, Oliveira S, Armbrust W, Garay S, Vojinovic J, Norambuena X, Gamir ML, García-Consuegra J, Lepore L, Susic G, Corona F, Dolezalova P, Pistorio A, Martini A, Ruperto N, Roth J (2010) Paediatric rheumatology international trials organization (PRINTO). Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA 303(13):1266–1273. Erratum in: JAMA. 2010 May 26;303(20):2034
Acknowledgments
We thank our SoJIA patients and families. We thank Prof. Dr. rer. nat. Sybille Bergmann (Department of Clinical Chemistry and Endocrinology, University Hospital “Carl Gustav Carus”, Dresden) for her support in serum cytokine analyses. We further thank the staff of University Children’s Hospital Dresden for taking care of our patients and Christine Hendrix for her contributions.
Conflict of interest
The authors (C.M. Hedrich, N. Bruck, B. Fiebig, and M. Gahr) state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedrich, C.M., Bruck, N., Fiebig, B. et al. Anakinra: A safe and effective first-line treatment in systemic onset juvenile idiopathic arthritis (SoJIA). Rheumatol Int 32, 3525–3530 (2012). https://doi.org/10.1007/s00296-011-2249-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-011-2249-4