Abstract
Histopathology plays an important role in defining response to treatment for different tumor types. Histopathologic response criteria are currently used as reference standard in various types of cancer, including breast cancer, gastroesophageal cancer, and bone tumors. Since there were no generally accepted response criteria established for ovarian cancer, a systematic analysis of various features of tumor regression was performed. Patient survival served as the reference standard to validate the histopathologic features of tumor regression. In contrast to ovarian cancer, borderline ovarian tumors are epithelial ovarian neoplasms characterized by up-regulated cellular proliferation and cytologic atypia but without destructive stromal invasion. While borderline ovarian tumors generally have an excellent prognosis with a 5‑year survival of > 95%, recurrences and malignant transformation occur in a small percentage of patients. Nevertheless, the identification of patients at increased risk for recurrence remains difficult. The aim of studying histopathological markers in ovarian cancers and borderline tumors was to evaluate whether histopathologic features including molecular pathologic alterations can predict patient outcome, particularly the risk of recurrence of serous and mucinous borderline tumors.
Zusammenfassung
Die Histopathologie spielt bei der Bestimmung des Therapieansprechens verschiedener Tumoren eine wichtige Rolle. Während die histopathologische Tumorregression als Goldstandard zur Evaluierung des Therapieansprechens für mehrere solide Tumoren, einschließlich Mamma- und Magenkarzinome sowie Osteosarkome, etabliert ist, existieren dazu keine gesicherten Kriterien. Diese Arbeit untersuchte histopathologische Regressionskriterien mit dem Gesamtüberleben der Patientinnen als Referenzstandard für Therapieansprechen. Im Gegensatz zu Ovarialkarzinomen weisen Borderline-Tumoren des Ovars generell eine sehr gute Prognose mit einem 5‑Jahres-Überleben von > 95% auf. Dennoch kommt es in einem kleinen Prozentsatz zu Rezidiven, teilweise auch mit maligner Transformation. Verlässliche Parameter zur Vorhersage des Rezidivrisikos fehlen weitgehend und könnten zu einer individualisierten Therapieentscheidung beitragen. Das Ziel der Arbeit war es zu evaluieren, ob histologische und molekularpathologische Kriterien zu einer verbesserten Vorhersage der Prognose und insbesondere des Rezidivrisikos von serösen und muzinösen Borderline-Tumoren beitragen.
Similar content being viewed by others
References
Avril S et al (2012) Histopathologic features of ovarian borderline tumors are not predictive of clinical outcome. Gynecol Oncol 127(3):516–524
Sassen S et al (2007) Histopathologic assessment of tumor regression after neoadjuvant chemotherapy in advanced-stage ovarian cancer. Hum Pathol 38(6):926–934
Avril-Sassen S et al (2009) Characterisation of microRNA expression in post-natal mouse mammary gland development. BMC Genomics 10:548
Raychaudhuri M et al (2012) Intratumoral Heterogeneity of MicroRNA Expression in Breast Cancer. J Mol Diagn 14(4):376–384
Malinowsky K et al (2012) Common protein biomarkers assessed by reverse phase protein arrays show considerable intratumoral heterogeneity in breast cancer tissues. PLOS ONE 7(7):e40285
Mittermeyer G et al (2013) Variation in cell signaling protein expression may introduce sampling bias in primary epithelial ovarian cancer. PLOS ONE 8(10):e77825
Becker K et al (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98(7):1521–1530
Karamitopoulou E et al (2014) Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol 38(11):1551–1556
Salzer-Kuntschik M, Brand G, Delling G (1983) Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe 4(3):135–141
Schmidt T et al (2014) Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 110(7):1712–1720
Sinn HP et al (1994) Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy. Geburtshilfe Frauenheilkd 54(10):552–558
Fisher ER et al (2002) Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B‑18. Cancer 95(4):681–695
Symmans WF et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422
Junker K et al (2001) Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest 120(5):1584–1591
McCluggage WG et al (2002) Morphological effects of chemotherapy on ovarian carcinoma. J Clin Pathol 55(1):27–31
Moreno A et al (2002) Pathologic changes related to CMF primary chemotherapy in breast cancer. Pathological evaluation of response predicts clinical outcome. Breast Cancer Res Treat 75(2):119–125
Heintz AP et al (2006) Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 95(Suppl 1):S161–92
Deutsche Krebsgesellschaft, AWMF (2013) S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren, Version 1.0. AWMF Registrierungsnummer: 032–035OL. http://leitlinienprogramm-onkologie.de/Leitlinien.7.0.html. Accessed January 2015
Cannistra SA (2004) Cancer of the ovary. N Engl J Med 351(24):2519–2529
Silverberg SG et al (2004) Borderline ovarian tumors: key points and workshop summary. Hum Pathol 35(8):910–917
Seidman JD, Kurman RJ (2000) Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol 31(5):539–557
Shih Ie M, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164(5):1511–1518
Singer G et al (2005) Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol 29(2):218–224
Kurman RJ, Shih Ie M (2008) Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 27(2):151–160
Kurman RJ, Shih Ie M (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34(3):433–443
Seidman JD et al (2004) Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol 35(8):918–933
Alvarez-Garcia I, Miska EA (2005) MicroRNA functions in animal development and human disease. Development 132(21):4653–4662
Farazi TA et al (2011) miRNAs in human cancer. J Pathol 223(2):102–115
Ma L, Weinberg RA (2008) MicroRNAs in malignant progression. Cell Cycle 7(5):570–572
Sassen S, Miska EA, Caldas C (2008) MicroRNA: implications for cancer. Virchows Arch 452(1):1–10
Doleshal M et al (2008) Evaluation and validation of total RNA extraction methods for microRNA expression analyses in formalin-fixed, paraffin-embedded tissues. J Mol Diagn 10(3):203–211
Hasemeier B et al (2008) Reliable microRNA profiling in routinely processed formalin-fixed paraffin-embedded breast cancer specimens using fluorescence labelled bead technology. BMC Biotechnol 8:90
Siebolts U et al (2009) Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J Clin Pathol 62(1):84–88
Lu J et al (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838
Rosenfeld N et al (2008) MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 26(4):462–469
Slack FJ, Weidhaas JB (2008) MicroRNA in cancer prognosis. N Engl J Med 359(25):2720–2722
Tavazoie SF et al (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451(7175):147–152
Yanaihara N et al (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9(3):189–198
Iorio MV et al (2005) MicroRNA gene expression deregulation in human breast cancer. Cancer Res 65(16):7065–7070
Blenkiron C et al (2007) MicroRNA expression profiling of human breast cancer identifies new markers of tumour subtype. Genome Biol 8(10):R214
Andorfer CA et al (2011) MicroRNA signatures: clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol Med 17(6):313–319
Esteva FJ, Hortobagyi GN (2004) Prognostic molecular markers in early breast cancer. Breast Cancer Res 6(3):109–118
Grubb RL et al (2003) Signal pathway profiling of prostate cancer using reverse phase protein arrays. Proteomics 3(11):2142–2146
Paweletz CP et al (2001) Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene 20(16):1981–1989
Espina V et al (2003) Protein microarrays: molecular profiling technologies for clinical specimens. Proteomics 3(11):2091–2100
Liotta LA et al (2003) Protein microarrays: meeting analytical challenges for clinical applications. Cancer Cell 3(4):317–325
Wulfkuhle JD et al (2006) Technology insight: pharmacoproteomics for cancer – promises of patient-tailored medicine using protein microarrays. Nat Clin Pract Oncol 3(5):256–268
Berg D et al (2011) Use of formalin-fixed and paraffin-embedded tissues for diagnosis and therapy in routine clinical settings. Methods Mol Biol 785:109–122
Wolff C et al (2011) Producing reverse phase protein microarrays from formalin-fixed tissues. Methods Mol Biol 785:123–140
Murphy CG, Modi S (2009) HER2 breast cancer therapies: a review. Biologics 3:289–301
Berx G, Van Roy F (2001) The E‑cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 3(5):289–293
Look MP et al (2002) Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst 94(2):116–128
Schmitt M et al (2008) Assessment of urokinase-type plasminogen activator and its inhibitor PAI-1 in breast cancer tissue: historical aspects and future prospects. Breast Care (Basel) 3(s2):3–10
Dean E, El-Helw L, Hasan J (2010) Targeted therapies in epithelial ovarian cancer. Cancers (Basel) 2(1):88–113
Moroney JW et al (2011) A phase I trial of liposomal doxorubicin, bevacizumab, and temsirolimus in patients with advanced gynecologic and breast malignancies. Clin Cancer Res 17(21):6840–6846
Dean E et al (2010) Targeted therapies in epithelial ovarian cancer. Cancers (Basel) 2(L):88–113
Crum CP (2009) Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol 3(2):165–170
Gross AL et al (2010) Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. J Oncol. https://doi.org/10.1155/2010/126295
Honkoop AH et al (1997) Effects of chemotherapy on pathologic and biologic characteristics of locally advanced breast cancer. Am J Clin Pathol 107(2):211–218
Mandard AM et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73(11):2680–2686
Junker K (2004) Therapy-induced morphological changes in lung cancer. Pathologe 25(6):475–480
du Bois A et al (2013) Borderline tumours of the ovary: A cohort study of the Arbeitsgmeinschaft Gynakologische Onkologie (AGO) Study Group. Eur J Cancer 49(8):1905–1914
Cadron I et al (2007) Management of borderline ovarian neoplasms. J Clin Oncol 25(20):2928–2937
Cusido M et al (2007) Results of the national survey of borderline ovarian tumors in Spain. Gynecol Oncol 104(3):617–622
Du Bois A et al (2009) Borderlinetumoren des Ovars – eine systematische Übersicht. Borderline Tumors of the Ovary – A Systematic Review. Geburtshilfe Frauenheilkd 69:1–27
Shih KK et al (2011) Accuracy of frozen section diagnosis of ovarian borderline tumor. Gynecol Oncol 123(3):517–521
Burks RT, Sherman ME, Kurman RJ (1996) Micropapillary serous carcinoma of the ovary. A distinctive low-grade carcinoma related to serous borderline tumors. Am J Surg Pathol 20(11):1319–1330
Seidman JD, Kurman RJ (1996) Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol 20(11):1331–1345
Prat J, De Nictolis M (2002) Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol 26(9):1111–1128
Ronnett BM et al (2004) Mucinous borderline ovarian tumors: points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum Pathol 35(8):949–960
Park JY et al (2011) Micropapillary pattern in serous borderline ovarian tumors: does it matter? Gynecol Oncol 123(3):511–516
Eichhorn JH et al (1999) Ovarian serous borderline tumors with micropapillary and cribriform patterns: a study of 40 cases and comparison with 44 cases without these patterns. Am J Surg Pathol 23(4):397–409
Bell DA et al (2004) Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum Pathol 35(8):934–948
Morice P et al (2003) Prognostic factors for patients with advanced stage serous borderline tumours of the ovary. Ann Oncol 14(4):592–598
Gershenson DM et al (1998) Ovarian serous borderline tumors with invasive peritoneal implants. Cancer 82(6):1096–1103
Kommission Ovar der AGO, Schmalfeldt B, Pfisterer J (eds) (2007) Interdisziplinäre S2 k‑Leitlinie für die Diagnostik und Therapie maligner Ovarialtumoren. W. Zuckschwerdt, München
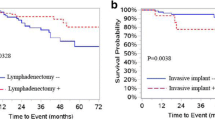
Shih KK et al (2011) Risk factors for recurrence of ovarian borderline tumors. Gynecol Oncol 120(3):480–484
Daniel CW, Smith GH (1999) The mammary gland: a model for development. J Mammary Gland Biol Neoplasia 4(1):3–8
Hennighausen L, Robinson GW (2005) Information networks in the mammary gland. Nat Rev Mol Cell Biol 6(9):715–725
Caldas C, Brenton JD (2005) Sizing up miRNAs as cancer genes. Nat Med 11(7):712–714
Greene SB et al (2010) A putative role for microRNA-205 in mammary epithelial cell progenitors. J Cell Sci 123(Pt 4):606–618
Ibarra I et al (2007) A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev 21(24):3238–3243
Sempere LF et al (2007) Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res 67(24):11612–11620
Farh KK et al (2005) The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 310(5755):1817–1821
Lim LP et al (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433(7027):769–773
Mattie MD et al (2006) Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer 5:24
Lowery AJ et al (2009) MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res 11(3):R27
Volinia S et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103(7):2257–2261
Farazi TA et al (2011) MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res 71(13):4443. https://doi.org/10.1158/0008-5472.CAN-11-0608
Heyn H et al (2011) MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. Int J Cancer 129(12):2797. https://doi.org/10.1002/ijc.25962
Camps C et al (2008) hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14(5):1340–1348
Ma L, Teruya-Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449(7163):682. https://doi.org/10.1038/nature06174 (Erratum in Nature. 2008 Sep 11;455(7210):256)
Nassar A et al (2010) Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: a tissue microarray-based study. Appl Immunohistochem Mol Morphol 18(5):433–441
Brunelli M et al (2009) Genotypic intratumoral heterogeneity in breast carcinoma with HER2/neu amplification: evaluation according to ASCO/CAP criteria. Am J Clin Pathol 131(5):678–682
Shin SJ et al (2006) Intratumoral heterogeneity of her-2/neu in invasive mammary carcinomas using fluorescence in-situ hybridization and tissue microarray. Int J Surg Pathol 14(4):279–284
Fujii H et al (1996) Genetic divergence in the clonal evolution of breast cancer. Cancer Res 56(7):1493–1497
Shen CY et al (2000) Genome-wide search for loss of heterozygosity using laser capture microdissected tissue of breast carcinoma: an implication for mutator phenotype and breast cancer pathogenesis. Cancer Res 60(14):3884–3892
Glockner S et al (2002) Marked intratumoral heterogeneity of c‑myc and cyclinD1 but not of c‑erbB2 amplification in breast cancer. Lab Invest 82(10):1419–1426
Lonn U et al (1994) Intratumoral heterogeneity for amplified genes in human breast carcinoma. Int J Cancer 58(1):40–45
Hall M et al (2013) Targeted anti-vascular therapies for ovarian cancer: current evidence. Br J Cancer 108(2):250–258
Wolff C et al (2011) Signalling networks associated with urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1 in breast cancer tissues: new insights from protein microarray analysis. J Pathol 223(1):54–63
Becker KF et al (2007) Quantitative protein analysis from formalin-fixed tissues: implications for translational clinical research and nanoscale molecular diagnosis. J Pathol 211(3):370–378
Berg D et al (2011) Protein microarray-based comparison of HER2, estrogen receptor, and progesterone receptor status in core biopsies and surgical specimens from FFPE breast cancer tissues. Appl Immunohistochem Mol Morphol 19(4):300–305
Hennessy BT et al (2010) A technical assessment of the utility of reverse phase protein arrays for the study of the functional proteome in non-microdissected human breast cancers. Clin Proteomics 6(4):129–151
Burger RA et al (2007) Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25(33):5165–5171
Cannistra SA et al (2007) Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25(33):5180–5186
Machida S et al (2005) Inhibition of peritoneal dissemination of ovarian cancer by tyrosine kinase receptor inhibitor SU6668 (TSU-68). Int J Cancer 114(2):224–229
Xu L et al (2000) Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol 16(3):445–454
Lee S et al (2005) Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol 97(1):26–34
Peng DJ et al (2010) Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Biophys Res Commun 394(3):600–605
Yang X et al (2006) Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res 66(6):3126–3136
Alaiya AA et al (1999) Two-dimensional gel analysis of protein expression in ovarian tumors shows a low degree of intratumoral heterogeneity. Electrophoresis 20(4–5):1039–1046
Rubin SC et al (1991) Analysis of antigen expression at multiple tumor sites in epithelial ovarian cancer. Am J Obstet Gynecol 164(2):558–563
Kobel M et al (2011) Biomarker expression in pelvic high-grade serous carcinoma: comparison of ovarian and omental sites. Int J Gynecol Pathol 30(4):366–371
Abelson S et al (2012) Intratumoral heterogeneity in the self-renewal and tumorigenic differentiation of ovarian cancer. Stem Cells 30(3):415–424
Zborovskaya I et al (1999) Somatic genetic alterations (LOH) in benign, borderline and invasive ovarian tumours: intratumoral molecular heterogeneity. Int J Cancer 82(6):822–826
Gerlinger M et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892
Acknowledgements
The studies summarized in this article were in part funded by Deutsche Forschungsgemeinschaft (DFG) Grant No SA1698/1–2 awarded to Stefanie Avril. The author would like to thank her mentors, Professors Heinz Hoefler, Markus Schwaiger, Marion Kiechle, Carlos Caldas, and Eric Miska, as well as collaborators and co-authors on the original publications of this work: Mithu Raychaudhuri and Theresa Buchner (heterogeneity of miRNAs in breast cancer), Karl-Friedrich Becker and Katharina Malinowsky (intratumoral heterogeneity of protein expression), Barbara Schmalfeldt and Holger Bronger (treatment response and recurrence risk in ovarian cancer and borderline tumors). Stefanie Avril is currently supported by the Clinical and Translational Science Collaborative of Cleveland (KL2TR000440) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Avril declares that she has no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry
Rights and permissions
About this article
Cite this article
Avril, S. Histopathological markers of treatment response and recurrence risk in ovarian cancers and borderline tumors. Pathologe 38 (Suppl 2), 180–191 (2017). https://doi.org/10.1007/s00292-017-0375-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00292-017-0375-9