Abstract
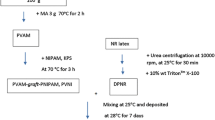
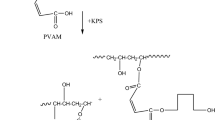
To date, smart hydrogels are widely used in the biomedical field such as wound dressings, artificial tendons, drugs and tissues owing to its sensitivity on environmental medium. The present work was focused on the preparation of a maleated poly(vinyl alcohol) (PVAM)-g-gelatin (GT) (PVAM-g-GT) from the PVAM and the GT in aqueous solutions using K2S2O8 as an initiator. The characteristic bands of the novel smart PVAM-g-GT hydrogel were observed with peaks at 3288, 1541 and 1238 cm−1 observed by FTIR. The increasing amounts of GT resulted in a decrease of the crystallinity. The swelling ratio in the water increased and the contact angle decreased with an increase of GT in the graft copolymer due to an increase of the hydrophilic groups in the hydrogel. In addition, the tensile strength of the novel smart PVAM-g-GT increased with an increase of the GT. The highest tensile strength and elongation at break were found at 8:2 PVAM:GT. Moreover, PVAM-g-GT shows a good responsibility to pH and temperature and it was used to a polymer matrix for encapsulation of capsaicin. A lower capsaicin release rate from the PVAM-g-GT hydrogel was obtained when immersed at pH 8 than at pH 2 due to hydrogen bond between the capsaicin and the polymer matrix.









Similar content being viewed by others
References
Cencetti C, Bellini D, Pavesio A, Senigaglia D, Passariello C, Virga A, Matricardi P (2012) Preparation and characterization of antimicrobial wound dressings based on silver, gellan, PVA and borax. Carbohydr Polym 90:1362
Kobayashi H, Kato Mtaguchi T, Ikoma T, Miyashita H, Shimmura S, Tsubota K, Tanaka J (2004) Collagen immobilized PVA hydrogel–hydroxyapatite composites prepared by kneading methods as a material for peripheral cuff of artificial. J Mater Sci Eng C 247:29
Saunders JR, Moussa W (2013) AC frequency-based electrical stimulation of hydrogel microactuators employing Parylene-N coated electrodes. Sens Actuators B 182:761
Fazly Bazzaz BS, Khameneh B, Jalili-Behabadi Malaekeh-Nikouei B, Mohajeri SA (2014) Preparation, characterization and antimicrobial study of a hydrogel (soft contact lens) material impregnated with silver nanoparticles. Contact Lens Anterior Eye 37:149
Maiti S, Mukherjee S (2012) Controlled drug delivery attributes of co-polymer micelles and xanthan-O-carboxymethyl hydrogel particles. Int J Biol Macromol 70:37
Chan K, Li Chapman J, Trac E, Kobler J, Zeitels S, Langer R, Karajanagi S (2014) Functionalizable hydrogel microparticles of tunable size and stiffness for soft-tissue filler applications. Acta Biomater 10:2563
Ye M, Mohanty P, Ghosh G (2014) Morphology and properties of poly vinyl alcohol (PVA) scaffolds: impact of process variables. Mater Sci Eng C 42:289
Lim KS, Alves MH, Martens PJ (2013) Covalent incorporation of non- chemically modified gelatin into degradable PVA-tyramine hydrogels. Biomaterial 34:7097
Hui B (2014) Zhang Y and Ye L (2014) Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. J Ind Eng Chem 235:207
Harisha A, Ravindrachary V, Bhajantri RF, Ismayil Sanjeev G, Poojary B, Dutta D, Pujari PK (2008) Electron irradiation induced microstructural modifications in BaCl2 doped PVA: a positron annihilation study. Polym Degrad Stabil 93:1554
Shang Y, Peng Y (2008) UF membrane of PVA modified with TDI. Desalination 221:324
Duflot AV, Kitaeva NK, Duflot VR (2015) Radiation-chemical preparation of poly(vinyl alcohol) hydrogels. Radiat Phys Chem 107:1
Kartal F, Akkaya A, Kilinc A (2009) Radiation-chemical preparation of poly(vinyl alcohol) hydrogels. J Mol Catal B Enzym 57:55
Liu Y, Chiu Y (2003) Covalent incorporation of non- chemically modified gelatin into degradable PVA-tyramine hydrogels. J Polym Sci Part A Polym Chem 41:107
Riyajan S, Sasithornsonti S (2013) Chemical crosslink degradable pva aqueous solution by potassium persulphate. J Polym Environ 21:472
Sukhlaaied W, Riyajan S (2014) Green synthesis and physical properties of poly(vinyl alcohol) maleated in an aqueous solutions. J Polym Environ 22:350
Prajapati VD, Maheriya PM, Jani GK, Solanki HK (2014) Carrageenan: a natural seaweed polysaccharide and its applications. Carbohydr Polym 105:97
Riyajan S, Sasithornsonti S, Phinyocheep P (2012) Green natural rubber-g-modified starch for controlling urea release. Carbohydr Polym 89:251
Yuliantia E, Karoa AK, Susitab L, Sudaryanto (2012) Synthesis of electrolyte polymer based on natural polymer chitosan by ion implantation technique. Proc Chem 4:202
Tamura H, Furuike T, Nair SV, Jayakumar R (2011) Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr Polym 84:820
Vieira DF, Pawlicka A (2010) Optimization of performances of gelatin/LiBF4-based polymer electrolytes by plasticizing effects. Electrochim Acta 55:1489
Dash R, Foston M, Ragauskas AJ (2013) Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr Polym 91:638
Linh NT, Min YK, Song H, Lee B (2010) Fabrication of polyvinyl alcohol/gelatin nanofiber composites and evaluation of their material properties. J Biomed Mate Res Part B 95:184
Kuila SB, Ray SK (2014) Dehydration of dioxane by pervaporation using filled blend membranes of polyvinyl alcohol and sodium alginate. Carbohydr Polym 101:1154–1165
Lv X, Jiang G, Xue X, Wu D, Sheng T, Sun C, Xu X (2013) Fe0-Fe3O4 nanocomposites embedded polyvinyl alcohol/sodium alginate beads for chromium (VI) removal. J Hazard Mater 262:748
Zhu Y, Peng W, Zhang J, Wang M, Firempong CK, Feng C, Liu H, Xu X, Yu J (2014) Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: preparation, in vitro and in vivo evaluation. J Funct Food 8:358
Liu M, Su H, Tan T (2012) Synthesis and properties of thermo- and pH-sensitive poly(N-isopropylacrylamide)/polyaspartic acid IPN hydrogels. Carbohydr Polym 87:2425
Sukhlaaied W, Riyajan S (2013) Synthesis and properties of carrageenan grafted copolymer with poly(vinyl alcohol). Carbohydr Polym 98:677
Antunes CF, van Duin M, Machado AV (2011) Morphology and phase inversion of EPDM/PP blends—effect of viscosity and elasticity. Polym Test 30:907–915
Bettaïeb NB, Karbowiak T, Bornaz S, Debeaufort F (2015) Spectroscopic analyses of the influence of electron beam irradiation doses on mechanical, transport properties and microstructure of chitosan-fish gelatin blend films. Food Hydrocolloid 46:37–51
Ma L, Liu M, Liu H, Chen J, Cui D (2010) In vitro cytotoxicity and drug release properties of pH- and temperature-sensitive core–shell hydrogel microspheres. Int J Pharm 385:86
Kulkarni RV, Boppana R, Mohan GK, Mutalik S, Kalyane NV (2012) pH-responsive interpenetrating network hydrogel beads of poly(acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: synthesis, in vitro and in vivo evaluation. J Colloid Interface Sci 367:509
Curcio M, Spizzirri UG, Iemma F, Puoci F, Cirillo G, Parisi OI, Picci N (2010) Grafted thermo-responsive gelatin microspheres as delivery systems in triggered drug release. Eur J Pharm Biopharm 76:48
Fan T, Li M, Wu X, Li M, Wu Y (2011) Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers. Colloids Surf B 88:593
Liu X, Guo H, Zha L (2012) Study of pH/temperature dual stimuli-responsive nanogels \with interpenetrating polymer network structure. Polym Int 61:1144
Bradley C, Jalili N, Nett SK, Chu L, Förch R, Gutmann J, Berger R (2009) Response characteristics of thermal responsive polymers using nanomechancial cantilever sensors. Macromol Chem Phys 210:1339
Wenceslau AC, Santos FG, Ramos ÉRF, Nakamura CV, Rubira AF, Muniz EC (2012) Thermo- and pH-sensitive IPN hydrogels based on PNIPAAm and PVA-Ma networks with LCST tailored close to human body temperature. Mater Sci Eng C 32:259
Riyajan S, Sakdakdapiphanich J (2010) Characterization of biodegradable semi-interpenetrating polymer based on poly(vinyl alcohol) and sodium alginate containing natural neem (Azadirachta indica) for controlled release application. Polym Int 59:1130
Acknowledgments
The authors thank the Department of Materials Science and Technology, Prince of Songkla University for the use of laboratory space, The Thailand Research Fund/Prince of Songkla University/Thammasat University for financial support (RSA5780018) and The Royal Golden Jubilee Ph.D. Program (2.L.PS/53/C.1.N.XX) and the author gratefully acknowledge the partial support provided by Central Instrument Center (CSIC), Faculty of Science and Technology, Thammasat University. Thanks to Dr. Brian Hodgson for assistance with the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukhlaaied, W., Riyajan, SA. Green robust pH–temperature-sensitive maleated poly(vinyl alcohol)-g-gelatin for encapsulated capsaicin. Polym. Bull. 73, 2303–2320 (2016). https://doi.org/10.1007/s00289-016-1609-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1609-3