Abstract
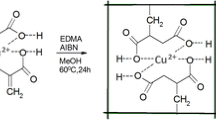
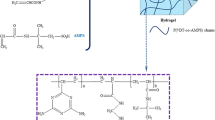
In the current study, in situ free radical polymerization was employed to prepare Cu2+-imprinted composite hydrogel (Cu2+-ICH). The cross-sectional morphology of the Cu2+-ICH evaluated by scanning electron microscopy indicated that the copper-loaded Cu2+-ICH became rougher and the pore size of the gel became smaller compared to the unloaded Cu2+-ICH. The ability of the Cu2+-ICH to adsorb Cu2+ from aqueous solutions was assessed using batch adsorption technique. The adsorption capacity increased with the initial concentration of Cu2+, but decreased as the temperature rose from 298 to 318 K. Thermodynamic parameters such as Gibbs free energy (ΔG 0), enthalpy (ΔH 0), and entropy (ΔS 0) for the Cu2+ adsorption were evaluated. It was suggested that the adsorption process was a spontaneous, exothermic process that had positive entropy. Selectivity study indicated that ion imprinting technique resulted in excellent affinity of the Cu2+-ICH toward Cu2+. Finally, the adsorption mechanism was studied by Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy. The results indicated that copper adsorption was mainly through interactions with the amine and carbonyl groups.








Similar content being viewed by others
References
Anirudhan TS, Suchithra PS (2010) Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: kinetics and equilibrium modeling. Chem Eng J 156:146–156
Chen JH, Liu QL, Hu SR, Ni JC, He YS (2011) Adsorption mechanism of Cu(II) ions from aqueous solution by glutaraldehyde crosslinked humic acid-immobilized sodium alginate porous membrane adsorbent. Chem Eng J 173:511–519
Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater 174:137–143
Ozsoy HD, Kumbur H, Saha B, van Leeuwen JH (2008) Use of Rhizopus oligosporus produced from food processing wastewater as a biosorbent for Cu(II) ions removal from the aqueous solutions. Bioresour Technol 99:4943–4948
Ucun H, Aksakal O, Yildiz E (2009) Copper(II) and zinc(II) biosorption on Pinus sylvestris L. J Hazard Mater 161:1040–1045
Karthikeyan S, Balasubramanian R, Iyer CSP (2007) Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol 98:452–455
Amarasinghe BMWPK, Williams RA (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132:299–309
Pourjavadi A, Amini-Fazl MS (2007) Optimized synthesis of carrageenan-graft-poly(sodium acrylate) superabsorbent hydrogel using the Taguchi method and investigation of its metal ion absorption. Polym Int 56:283–289
Wang J, Liu F, Wei J (2011) Enhanced adsorption properties of interpenetrating polymer network hydrogels for heavy metal ion removal. Polym Bull 67:1709–1720
Jeria-Orell M, Pizarro GDC, Marambio OG, Geckeler KE (2009) Novel hydrogels based on itaconic acid and citraconic acid: synthesis, metal ion binding, and swelling behavior. J Appl Polym Sci 113:104–111
Essawy HA, Ibrahim HS (2004) Synthesis and characterization of poly(vinylpyrrolidone-co-methylacrylate) hydrogel for removal and recovery of heavy metal ions from wastewater. React Funct Polym 61:421–432
Ji X, Hampsey JE, Hu Q, He J, Yang Z, Lu Y (2003) Mesoporous silica-reinforced polymer nanocomposites. Chem Mater 15:3656–3662
Sanchez C, Soler-Illia GJAA, Ribot F, Lalot T, Mayer CR, Cabuil V (2001) Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem Mater 13:3061–3083
Wang J, Li X (2013) Ion-imprinted composite hydrogels with excellent mechanical strength for selective and fast removal of Cu2+. Ind Eng Chem Res 52:572–577
Ahmadi SJ, Noori-Kalkhoran O, Shirvani-Arani S (2010) Synthesis and characterization of new ion-imprinted polymer for separation and preconcentration of uranyl (UO2 2+) ions. J Hazard Mater 175:193–197
Singh DK, Mishra S (2010) Synthesis and characterization of Hg(II)-ion-imprinted polymer: kinetic and isotherm studies. Desalination 257:177–183
Tarley CRT, Andrade FN, de Santana H, Zaia DAM, Beijo LA, Segatelli MG (2012) Ion-imprinted polyvinylimidazole-silica hybrid copolymer for selective extraction of Pb(II): characterization and metal adsorption kinetic and thermodynamic studies. React Funct Polym 72:83–91
Singh DK, Mishra S (2010) Synthesis and characterization of Hg(II)-ion-imprinted polymer: kinetic and isotherm studies. Desalination 257:177–183
Shamsipur M, Besharati-Seidani A (2011) Synthesis of a novel nanostructured ion-imprinted polymer for very fast and highly selective recognition of copper(II) ions in aqueous media. React Funct Polym 71:131–139
Wang J, Li X (2015) Synthesis of polyacrylamide/modified silica composite hydrogels for synergistic complexation of heavy metal ions. Desalin Water Treat 53:230–237
Sun S, Wang A (2006) Adsorption properties of carboxymethyl-chitosan and cross-linked carboxymethyl-chitosan resin with Cu(II) as template. Sep Purif Technol 49:197–204
Wu J, Yu HQ (2006) Biosorption of 2,4-dichlorophenol from aqueous solution by phanerochaete chrysosporium biomass: isotherms, kinetics and thermodynamics. J Hazard Mater 137:498–508
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Purif Technol 35:223–240
Li Q, Chai L, Qin W (2012) Cadmium(II) adsorption on esterified spent grain: equilibrium modeling and possible mechanisms. Chem Eng J 197:173–180
Wang J, Li X (2010) Preparation and characterization of interpenetrating polymer network silicone hydrogels with high oxygen permeability. J Appl Polym Sci 116:2749–2757
Liu H, Yang F, Zheng Y, Kang J, Qu J, Chen JP (2011) Improvement of metal adsorption onto chitosan/Sargassum sp. composite sorbent by an innovative ion-imprint technology. Water Res 45:145–154
Jin L, Bai R (2002) Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 18:9765–9770
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Project 21407125), Science And Technology Project from the Ministry of Housing and Urban–Rural Development of the People’s Republic of China (2014-K7-007), Jiangsu Provincial Natural Science Foundation (No. BK2012251), open project from Key Laboratory for Ecological–Environment Materials of Jiangsu Province (No. EML201202), and research fund of Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province (No. AE201069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Li, J. Cu2+ adsorption onto ion-imprinted composite hydrogels: thermodynamics and mechanism studies. Polym. Bull. 72, 2143–2155 (2015). https://doi.org/10.1007/s00289-015-1394-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1394-4