Abstract
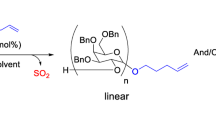
To investigate the relationship between the steric structures and polymerizability of five-membered cyclic carbonates, the anionic ring-opening polymerizations of methyl 4,6-O-benzylidene-2,3-O-carbonyl-α-d-galacto- and mannopyranosides (MBCGa and MBGM, respectively), were examined and compared to the results of the already reported glucopyranoside analog (MBCG). The polymerization of MBCGa proceeded without the elimination of CO2 as in the case of MBCG, while MBCM was not polymerized. To estimate the thermodynamic stability of the carbonate rings of these cyclic carbonates, the model reactions using the corresponding hydroxycarbonates were carried out. The ring-closing reactions of the mannopyranoside-based hydroxycarbonate proceeded to produce cyclic carbonate, while the others did not give cyclic carbonates. This should indicate that the carbonate rings of MBCG and MBCGa were less stable than that of MBCM. The single-crystal X-ray structural analysis indicated that the carbonate rings of MBCG and MBCGa have higher angle strains than that of MBCM. This should suggest that the ring strain of the five-membered cyclic carbonate increases by trans-fusing to the pyranose ring.






Similar content being viewed by others
References
Wang H, Dong JH, Qiu KY, Gu ZW (1998) Synthesis of poly(1,4-dioxan-2-one-co-trimethylene carbonate) for application in drug delivery systems. J Polym Sci A Polym Chem 36:1301–1307. doi:10.1002/(SICI)1099-0518(199806)36:8<1301:AID-POLA13>3.0.CO;2-A
Zhu KJ, Hendren RW, Jensen K, Pitt CG (1991) Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate). Macromolecules 24:1736–1740. doi:10.1021/ma00008a008
Lagisetti C, Urbansky M, Coates RM (2007) The dioxanone approach to (2S,3R)-2-C-methylerythritol 4-phosphate and 2,4-cyclodiphosphate, and various MEP analogues. J Org Chem 72:9886–9895. doi:10.1021/jo0711900
Mikami K, Lonnecker AT, Gustafson TP, Zinnel NF, Pai PJ, Russell DH, Wooley KJ (2013) Polycarbonates derived from glucose via an organocatalytic approach. J Am Chem Soc 135:6826–6829. doi:10.1021/ja402319m
Yokoe M, Aoi K, Okada M (2003) Biodegradable polymers based on renewable resources. VII. Novel random and alternating copolycarbonates from 1,4:3,6-dianhydrohexitols and aliphatic diols. J Polym Sci A Polym Chem 41:2312–2321. doi:10.1002/pola.10772
Yokoe M, Aoi K, Okada M (2005) Biodegradable polymers based on renewable resources. IX. Synthesis and degradation behavior of polycarbonates based on 1,4:3,6-dianhydrohexitols and tartaric acid derivatives with pendant functional groups. J Polym Sci A Polym Chem 43:3909–3919. doi:10.1002/pola.20830
Tian H, Tang Z, Zhuang X, Chen X, Jing X (2012) Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci 37:237–280. doi:10.1016/j.progpolymsci.2011.06.004
Tempelaar S, Mespouille L, Coulembier O, Dubois P, Dove AP (2013) Synthesis and post-polymerisation modifications of aliphatic poly(carbonate)s prepared by ring-opening polymerisation. Chem Soc Rev 42:1312–1336. doi:10.1039/c2cs35268k
Lu X-B, Ren W-M, Wu G-P (2012) CO2 copolymers from epoxides: catalyst activity, product selectivity, and stereochemistry control. Acc Chem Res 45:1721–1735. doi:10.1021/ar300035z
Omae I (2012) Recent developments in carbon dioxide utilization for the production of organic chemicals. Coord Chem Rev 256:1384–1405. doi:10.1016/j.ccr.2012.03.017
Klaus S, Lehenmeier MW, Anderson CE, Rieger B (2011) Recent advances in CO2/epoxide copolymerization—new strategies and cooperative mechanisms. Coord Chem Rev 255:1460–1479. doi:10.1016/j.ccr.2010.12.002
Rokicki G (2000) Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prog Polym Sci 25:259–342. doi:10.1016/S0079-6700(00)00006-X
Clements JH (2003) Reactive applications of cyclic alkylene carbonates. Ind Eng Chem Res 42:663–674. doi:10.1021/ie020678i
Haba O, Tomizuka H, Endo T (2005) Anionic ring-opening polymerization of methyl 4,6-O-benzylidene-2,3-O-carbonyl-α-d-glucopyranoside: a first example of anionic ring-opening polymerization of five-membered cyclic carbonate without elimination of CO2. Macromolecules 38:3562–3563. doi:10.1021/ma0476745
Azechi M, Matsumoto K, Endo T (2013) Anionic ring-opening polymerization of a five-membered cyclic carbonate having a glucopyranoside structure. J Polym Sci A Polym Chem 51:1651–1655. doi:10.1002/pola.26538
Tezuka K, Komatsu K, Haba O (2013) The anionic ring-opening polymerization of five-membered cyclic carbonates fused to the cyclohexane ring. Polym J 45:1183–1187. doi:10.1038/pj.2013.50
Haba O, Furuichi N, Akashika Y (2009) Anionic ring-opening copolymerization of l-lactide with a five-membered cyclic carbonate having a glucopyranoside structure. Polym J 41:702–708. doi:10.1295/polymj.PJ2008327
Duda A, Kowalski A (2009) Thermodynamics and kinetics of ring-opening polymerization. In: Dubois P, Coulembier O, Raquez JM (ed) Handbook of ring-opening polymerization, Wiley-VCH, Weinheim, pp 1–51. doi:10.1002/9783527628407.ch1
Haba O, Itabashi H (2014) Ring-opening polymerization of a five-membered lactone trans-fused to a cyclohexane ring. Polym J 46:89–93. doi:10.1038/pj.2013.70
Doane WM, Shasha BS, Stout EI, Russell CR, Rist CE (1967) A facile route to trans cyclic carbonates of sugars. Carbohydr Res 4:445–451. doi:10.1016/S0008-6215(00)81835-2
Kalikanda J, Li Z (2011) Study of the stereoselectivity of 2-azido-2-deoxygalactosyl donors: relationship to the steric factors of glycosyl acceptors. Carbohydr Res 346:2380–2383. doi:10.1016/j.carres.2011.08.015
Bebault GM, Dutton GGS (1974) Synthesis of 4-O-β-d-mannopyranosyl-l-rhamnopyranose. Carbohydr Res 37:309–319. doi:10.1016/S0008-6215(00)82920-1
Tewson TJ, Soderlind M (1985) 1-propenyl 4,6-O-Benzylidene-β-d-mannopyranoside-2,3-cyclic sulfate: a substrate for the synthesis of [F-18] 2-deoxy-2-fluoro-d-glucose. J Carbohydr Chem 4:529–543. doi:10.1080/07328308508082675
Sheldrick GM (2007) A short history of SHELX. Acta Cryst A64:112–122. doi:10.1107/S0108767307043930
Kabuto C, Akine S, Kwon E (2009) Release of software Yadokari-XG 2009 for crystal structure analyses. J Cryst Soc Jpn 51:201–204
Dainton FS, Ivin KJ (1958) Some thermodynamic and kinetic aspects of addition polymerisation. Q Rev Chem Soc 12:61–92. doi:10.1039/qr9581200061
Brown CJ (1954) The crystal structure of ethylene carbonate. Acta Cryst 7:92–96. doi:10.1107/S0365110X54000175
Pihlaja K, Rossi K (1977) Conformational analysis. Part XLIII. 13C chemical shifts and coupling constants as proof of the nonplanarity of the 2-oxo-1,3-dioxolane ring. Acta Chem Scand 31B:899–902. doi:10.3891/acta.chem.scand.31b-0899
Betz R, Klüfers P, Reichvilser MM (2007) 3,4-O-Carbonyl-1,2:5,6-di-O-isopropylidene-d-mannitol. Acta Cryst E63:o3890. doi:10.1107/S1600536807041244
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
289_2014_1295_MOESM1_ESM.pdf
Supplementary material 1 (PDF 782 kb) Supplementary data. Crystallographic data for structural analysis have been deposited with Cambridge Crystallographic Centre, CCDC No. 979619 for MBCG, No. 979620 for MBCGa, and No. 979621 for MBCM. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www: http://ccdc.cam.uk)
Rights and permissions
About this article
Cite this article
Tezuka, K., Koda, K., Katagiri, H. et al. Anionic ring-opening polymerization of five-membered cyclic carbonates derived from aldohexopyranosides. Polym. Bull. 72, 615–626 (2015). https://doi.org/10.1007/s00289-014-1295-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1295-y