Abstract
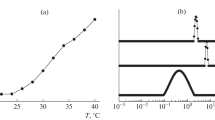
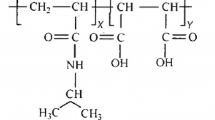
The field of thermo- and pH-sensitive copolymers, with applications in controlled drug delivery, is enriched with the copolymer based on N-vinylcaprolactam and maleic acid (VCL–MAc) that is synthesized and characterized in this paper. Cloud point measurements and dynamic light scattering are used to characterize the phase transition of VCL–MAc copolymers with variable composition in aqueous solution. The lower critical solution temperature (LCST) increases when the maleic acid content in the copolymer rises. Also, the LCST strongly depends on the polymer concentrations and the pH of the aqueous solution. The influence of the pH, and as a result of the polyelectrolyte dissociation, on the macromolecular chains conformation is studied by viscometric measurements. An interplay between Coulombic, hydrophobic and hydrogen bonding interactions determines the conformation of the macromolecules in solution and also the phase separation of VCL–MAc copolymers.










Similar content being viewed by others
Abbreviations
- CP:
-
Cloud point
- Dh:
-
Hydrodynamic diameter
- DLS:
-
Dynamic light scattering
- KPS:
-
Potassium persulfate
- LCST:
-
Lower critical solution temperature
- MAc:
-
Maleic acid
- OD450 :
-
Optical density at 450 nm
- VCL–MAc:
-
Vinylcaprolactam–maleic acid copolymer
References
Bawa P, Pillay V, Choonara YE, du Toit LC (2009) Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater 4:022001–022015. doi:10.1088/1748-6041/4/2/02200
Buengera D, Topuza F, Groll J (2012) Hydrogels in sensing applications. Prog Polym Sci 37:1678–1719. doi:10.1016/j.progpolymsci.2012.09.001
Lau ACW, Wu C (1999) Thermally sensitive and biocompatible poly(N-vinylcaprolactam): synthesis and characterization of high molar mass linear chains. Macromolecules 32:581–584. doi:10.1021/ma980850n
Laukkanen A, Valtola L, Winnik FM, Tenhu H (2004) Formation of colloidally stable phase separated poly(N-vinylcaprolactam) in water: a study by dynamic light scattering, microcalorimetry, and pressure perturbation calorimetry. Macromolecules 37:2268–2274. doi:10.1021/ma035124l
Maeda Y, Nakamura T, Ikeda I (2002) Hydration and phase behavior of poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone) in water. Macromolecules 35:217–222. doi:10.1021/ma011034
Dubovik AS, Machaeva EE, Grinberg VY, Khokhlov AR (2005) Energetics of cooperative transitions of N-vinylcaprolactam polymers in aqueous solutions. Macromol Chem Phys 206:915–928. doi:10.1002/macp.200400554
Li W, Wu P (2014) On the thermodinamic phase behavior of poly(N-vinylcaprolactam) solution in the presence of different ionic liquids. Polym Chem 5:761–770. doi:10.1039/C3PY01104F
Shtanko NI, Lequieu W, Goethlas EJ, du Prez FE (2003) pH- and thermo-responsive properties of poly(N-vinylcaprolactam-co-acrylic acid) copolymers. Polym Int 52:1605–1610. doi:10.1002/pi.1347
Makhaeva EE, Tenhu H, Khokhlov AR (2002) Behavior of poly(N-vinylcaprolactam-co-methacrylic acid) macromolecules in aqueous solution: interplay between coulombic and hydrophobic interaction. Macromolecules 35:1870–1876. doi:10.1021/ma0105789
Okhapkin IM, Nasimova IR, Makhaeva EE, Khokhlov AR (2003) Effect of complexation of monomer units on pH- and temperature-sensitive properties of poly(N-vinylcaprolactam-co-methacrylic acid). Macromolecules 36:8130–8138. doi:10.1021/ma035114k
Cao Y, He W (2011) Functionalized biocompatible poly(N-vinyl-2-caprolactam) with pH-dependent lower critical solution temperature behaviors. Macromol Chem Phys 212:2503–2510. doi:10.1002/macp.201100414
Tang S, Cao Y, Goddard SC, He W (2014) Synthesis of 3-(tert-butoxycarbonylmethyl)-N-vinyl-2-caprolactam and homologous copolymerization toward biocompatible carboxylated poly(N-vinyl-2-caprolactam) responsive to pH and temperature. J Polym Sci Part A: Polym Chem 52:112–120. doi:10.1002/pola.26977
Maeda Y, Yamamoto H, Ikeda I (2001) Effects of ionization of incorporated imidazole groups on the phase transitions of poly(N-isopropylacrylamide), poly(N, N-diethylacrylamide), and poly(N-vinylcaprolactam) in water. Langmuir 17:6855–6859. doi:10.1021/la0106438
Shatalov GV, Verezhnikov VN, Plaksitskaya TV, Kuznetzov VA, Poyarkova TN, Yan’shina AV (2006) Synthesis of N, N-dimethylaminoethyl methacrylate copolymers with N-vinyl caprolactam and their complexing and flocculating behavior. Polym Sci Ser A 48:563–568. doi:10.1134/S0965545X06060022
Liang X, Kozlovskaya V, Chen Y, Zavgorodna O, Kharlampieva E (2012) Thermosensitive multilayer hydrogels of poly(N-vinylcaprolactam) as nanothin films and shaped capsules. Chem Mater 24:3707–3719. doi:10.1021/cm301657q
Pich A, Tessier A, Boyo V, Lu Y, Adler H-JP (2006) Synthesis and characterization of poly(vinylcaprolactam)-based microgels exhibiting temperature and pH-sensitive properties. Macromolecules 39:7701–7707. doi:10.1021/ma060985q
Schachschal S, Balaceanu A, Melian C, Demco DE, Eckert T, Richtering W, Pich A (2010) Polyampholyte microgels with anionic core and cationic shell. Macromolecules 43:4331–7339. doi:10.1021/ma100184h
Imaz A, Forcada J (2011) Synthesis strategies to incorporate acrylic acid into N-vinylcaprolactam-based microgels. J Polym Sci Part A: Polym Chem 49:3218–3227. doi:10.102/pola.24758
Rao KM, Mallikarjuna B, Rao KSVK, Siraj S, Rao KC, Subha MCS (2013) Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surf, B 102:891–897. doi:10.1016/j.colsurfb.2012.09.009
Ilavský M, Mamytbekov G, Bouchal K, Hanyková L (1999) Phase transition in swollen gels 27. Effect of negative charge concentration on swelling and mechanical behaviour of poly(N-vinylcaprolactam) gels. Polym Bull 43:109–116
Sauvage E, Amos DA, Antalek B, Schroeder KM, Tan JS, Plucktaveesah N (2004) Amphiphilic maleic acid-containing alternating copolymers—1. Dissociation behavior and compositions. J Polym Sci Pol Phys 42:3571–3583. doi:10.1002/polb.20202
Popescu I, Suflet DM, Pelin IM, Popa MI (2012) Influence of the comonomer on the dissociation of some alternating maleic acid copolymers. J Macromol Sci Part B-Phys 51:1–15. doi:10.1080/00222348.2011.609784
Kesim H, Rzaev ZMO, Dinçer S, Pisşkin E (2003) Functional bioengineering copolymers. II. Synthesis and characterization of amphiphilic poly(N-isopropyl acrylamide-co-maleic anhydride) and its macrobranched derivatives. Polymer 44:2897–2909. doi:10.1016/S0032-3861(03)00177-0
Weiss-Malik RA, Solis FJ, Vernon BL (2004) Independent control of lower critical solution temperature and swelling behavior with pH for poly(N-isopropylacrylamide-co-maleic acid. J Appl Polym Sci 94:2110–2116. doi:10.1002/app.21000
Popescu I, Suflet DM, Pelin IM, Chitanu GC (2011) Biomedical applications of maleic anhydride copolymers. Rev Roum Chim 56:173–188
Azori M (1988) Polymeric prodrugs systems based on poly(N-vinylpyrrolidone-alt-maleic anhydride). In: Migliaresi C, Nicolais L, Guisti P, Chiellini E (eds) Poymers in medicine III: proceedings of the Third International Conference on Polymers in Medicine, Porto Cervo, Italy, June 9–13, 1987. Elsevier Science Publishers B. V., Amsterdam, pp 189–199
Can HK, Doğan AL, Rzaev ZMO, Uner AH, Güner A (2006) Synthesis, characterization, and antitumor activity of poly(maleic anhydride-co-vinyl acetate-co-acrylic acid). J Appl Polym Sci 100:3425–3432. doi:10.1002/app.21834
Karakus G, Zengin HB, Polat ZA, Yenidunya AF, Aydin S (2013) Cytotoxicity of three maleic anhydride copolymers and common solvents used for polymer solvation. Polym Bull 70:1591–1612. doi:10.1007/s00289-012-0860-5
Boghina C (1977) Copolymerization of N-vinyl-ε-caprolactam with maleic anhydride. Makromol Chem 178:1039–1047
Güven G, Rzaev ZMO (2008) Complex-radical copolymerization of N-vinyl pyrrolidone with isostructural analogs of maleic anhydride. Polym Bull 60:741–752. doi:10.1007/s00289-008-0909-7
Liu T, Chen J, Sugihara S, Maeda Y (2012) Study on hydration of poly(N-vinylcaprolactam) microgels by near-IR and mid-IR spectroscopy. Colloid Polym Sci 290:763–767. doi:10.1007/s00396-012-2621-2
Sun S, Wu P (2011) Infrared spectroscopic insight into hydration behavior of poly(N-vinylcaprolactam) in water. J Phys Chem B 115:11609–11618. doi:10.1021/jp2071056
Mȕller M, Kessler B, Richter S (2005) Preparatio of monomodal polyelectrolyte complex nanoparticles of PDADMAC/poly(maleic acid-alt-α-methylstyrene) by consecutive centrifugation. Langmuir 21:7044–7051. doi:10.1021/la050716d
Tripto E, Quinn JF, Caruso F (2005) Assembly of multilayer films from polyelectrolytes containing weak and strong acid moieties. Langmuir 21:8785–8792. doi:10.1021/la051197h
Spěváček J, Dybal J, Starovoytova L, Zhigunov A, Sedláková Z (2012) Temperature-induced phase separation and hydration in poly(N-vinylcaprolactam) aqueous solutions: a study by NMR and IR spectroscopy, SAXS, and quantum-chemical calculations. Soft Matter 8:6110–6119
Lozinsky VI, Simenel IA, Kulakova VK et al (2003) Synthesys and studies of N-vinylcaprolactam/N-vinylimidazole copolymers that exhibit the “proteinlike” behavior in aqueous media. Macromolecules 36:7308–7323. doi:10.1021/ma034456n
Balaceanu A, Demco DE, Moler M, Pich A (2011) Microgel heterogeneous morphology reflected in temperature-induced volume transition and 1H high-resolution transverse relaxation NMR. The case of poly(N-vinylcaprolactam)microgel. Macromolecules 44:2161–2169. doi:10.1021/ma200103y
Meeussen F, Nies E, Berghmans H, Verbrugghe S, Goethals E, du Prez F (2000) Phase behavior of poly(N-vinyl caprolactam) in water. Polymer 41:8597–8602. doi:10.1016/S0032-3861(00)00255-X
Delben F, Paoletti S, Prasso RD, Benegas JC (2006) Potentiometric titration of maleic acid copolymers in dilute aqueous solution: experimental results and theoretical interpretation. Macromol Chem Phys 207:2299–2310. doi:10.1002/macp.200600479
Reinhardt S, Steinert V, Werner K (1996) Investigations on the dissociation behavior of hydrolyzed alternating copolymers of maleic anhydride and propene-II. Viscometric behavior. Eur Polym J 32:939–942. doi:10.1016/0014-3057(96)00029-8
Kawaguchi S, Kitano T, Ito K (1991) Infrared and ultraviolet spectroscopic studies on intramolecular hydrogen bonding in an alternating copolymer of isobutylene and maleic acid. Macromolecules 24:3036–6030. doi:10.1021/ma00022a020
Hirose Y, Onodera M, Kawaguchi S, Ito K (1995) Monte Carlo simulation studies of conformational properties of polyelectrolytes with maleic acid units. Polym J 27:519–528
Yin X, Hoffman AS, Stayton P (2006) Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 7:1381–1385. doi:10.1021/bm0507812
Acknowledgments
This work was supported by a grant of the Romanian Ministry of Education, CNCS–UEFISCDI, project number PN-II-RU-PD-2012-3–0059. A.I. Prisacaru acknowledges the financial support from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 264115—STREAM. Paper dedicated to the 65th anniversary of “Petru Poni” Institute of Macromolecular Chemistry of Romanian Academy, Iasi, Romania.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popescu, I., Prisacaru, A.I., Suflet, D.M. et al. Thermo- and pH-sensitivity of poly(N-vinylcaprolactam-co-maleic acid) in aqueous solution. Polym. Bull. 71, 2863–2880 (2014). https://doi.org/10.1007/s00289-014-1227-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1227-x