Abstract
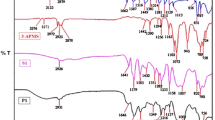
Two Schiff base polymers were prepared from the respective monomers by condensation method using toluene and N-methyl-2-pyrrolidinone as solvent. They were complexed with Al(III), In(III), and Cu(II) trifluoromethane sulfonates (triflates), and AlCl3 Lewis acids and characterized by FT-IR, UV–Vis spectroscopy, and scanning electron microscopy (SEM). Homogeneous films were prepared by spin coating in the presence and absence of Lewis acids. Polymer–Lewis acid interaction was confirmed by FT-IR, UV–Vis spectroscopy, and SEM. Lewis acid composition in polymers was determined by FT-IR spectroscopy. Absorption spectra of these conjugated Schiff base polymer complexes exhibited smaller optical band gap than pristine polymers. These variations ranged from 2.4 to 1.4 and 3.3 to 2.0 eV. Absorption depends on the Lewis acid in the polymer and band gap on the nature of the metal incorporated in the polymeric backbone. Solubility increased by complexation. The obtained complexes were soluble in trifluoroacetic and formic acids and in m-cresol. Polymer–Lewis acid solutions in m-cresol were stable for 98 h; the others remained stable over several months. The results of this study revealed that optical, solubility, and band-gap properties of conjugated Schiff base polymers can be modified by Lewis acids and these could be studied by optoelectronics.








Similar content being viewed by others
References
Bhadra S, Khastgir D, Singha NK, Lee JH (2009) Progress in preparation, processing and applications of polyaniline. Prog Polym Sci 34:783–810
Ramaprasad AT, Rao V (2010) Chitin–polyaniline blend as humidity sensor. Sens Actuators B Chem 148:117–125
Dhand C, Das M, Datta M, Malhotra BD (2011) Recent advances in polyaniline based biosensors. Biosens Bioelectron 26:2811–2821
Wang Q, J-l Li, Gao F, Li W-S, Wu K-Z (2008) Activated carbon coated with polyaniline as an electrode material in supercapacitors. New Carbon Mater 23:275–280
Madden JD, Rinderknecht D, Anquetil PA, Hunter IW (2007) Creep and cycle life in polypyrrole actuators. Sens Actuators A Phys 133:210–217
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196:1–12
Nogueira AF, Longo C, De Paoli MA (2004) Polymers in dye sensitized solar cells: overview and perspectives. Coord Chem Rev 248(13–14):1455–1468
Choi M-C, Kim Y, Ha C-S (2008) Polymers for flexible displays: from material selection to device applications. Prog Polym Sci 33:581–630
Gazotti WA, Nogueira AF, Girotto EM, Micaroni L, Martini M, das Neves S, De Paoli MA (2001) Optical devices based on conductive polymers. In: Nalwa HS (ed) Handbook of advanced electronic and photonic materials and devices, vol 10. Academic Press, San Diego, pp 53–98
Krinichnyi VI, Yudanova EI (2011) Structural effect of electron acceptor on charge transfer in poly(3-hexylthiophene)/methanofullerene bulk heterojunctions. Sol Energy Mater Sol Cells 95(8):2302–2313
Chochos CL, Choulis SA (2011) How the structural deviations on the backbone of conjugated polymers influence their optoelectronic properties and photovoltaic performance. Prog Polym Sci 36(10):1326–1414
Long Y-Z, Li M-M, Gu C, Wan M, Duvail J-L, Liu Z, Fan Z (2011) Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog Polym Sci 36(10):1415–1442
Bejbouji H, Vignau L, Miane JL, Dang M-T, Oualim M, Harmouchi M, Mouhsen A (2010) Polyaniline as a hole injection layer on organic photovoltaic cells. Sol Energy Mater Sol Cells 94(2):176–181
Sata T (1994) Properties of composite membranes prepared from ion exchange membranes and conducting polymers V. Dependence of EMF of composite membranes prepared from anion exchange membranes and polypyrrole on relative humidity. J Membr Sci 91(1–2):199–207
Li R, Lixin W, Haoyuan A, Fuqiang Z (2007) Study of lithium/polypyrrole secondary batteries with Lithium as cathode and polypyrrole anode. Rare Met 26(6):591–600
Schuhmann W, Lammert R, Hämmerle M, Schmidt H-L (1991) Electrocatalytic properties of polypyrrole in amperometric electrodes. Biosens Bioelectron 6(8):689–697
Belo D, Almeida M (2010) Transition metal complexes based on thiophene-dithiolene ligands. Coord Chem Rev 254(13–14):1479–1492
Roncali J (1997) Synthetic principles for bandgap control in linear π-conjugated systems. Chem Rev 97:173–205
Bundgaard E, Krebs FC (2007) Low band gap polymers for organic photovoltaics. Sol Energy Mater Sol Cells 91(11):954–985
Niu H-J, Huang Y-D, Bai X-D, Li X (2004) Novel poly-Schiff bases containing 4,4′-diamino-triphenylamine as hole transport material for organic electronic device. Mater Lett 58(24):2979–2983
Clarke TM, Durrant JR (2010) Charge photogeneration in organic solar cells. Chem Rev 110(11):6736–6767
Chidichimo G, Filippelli L (2010) Organic solar cells: problems and perspectives. Int J Photoenergy 2010, Article ID 123534. doi:10.1155/2010/123534
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109(11):5868–5923
Bernede JC (2008) Organic photovoltaic cells: history, principle and techniques. J Chil Chem Soc 53(3):1549–1564
Kietzke T (2007) Recent advances in organic solar cells. Adv OptoElectron 2007, Article ID 40285. doi:10.1155/2007/40285
Gunes S, Saricifci NS (2007) Conjugated polymer-based organic solar cells. Chem Rev 107:1324
Brabec CJ, Sariciftci N, Hummelen JC (2001) Plastic solar cells. Adv Funct Mater 11(1):15–26
Lee KS, Won JI, Jung JC (1989) Synthesis and characterization of processable conducting polyazomethines. Makromol Chem 190:1547–1552
Lee KS, Samoc M (1991) Third-order nonlinear optical properties of wholly aromatic polyazomethines. Polym Commun 32:361–362
Banerjee S, Gutch P, Saxena CJ (1995) Polyether azomethines. I. Synthesis and characterization. Polym Sci Part A 33(10):1719–1725
Banerjee S, Gutch PK, Saxena CJ (1999) Poly-Schiff bases-IV. Synthesis and characterization of poly(etherazomethine)s. Des Monomers Polym 2(2):135–142
Yang CJ, Jenekhe SA (1991) Conjugated aromatic poly(azomethines). Characterization of structure, electronic spectra, and processing of thin films from soluble complexes. Chem Mater 3(5):878–883
Yang CJ, Jenekhe SA (1995) Conjugated aromatic polyimines. 2. Synthesis, structure and properties of new aromatic polyazomethines. Macromolecules 28(4):1180–1185
Kim JY, Kim SH, Lee H-H, Ma W, Gong X (2006) New architecture for high-efficiency polymer photovoltaic cells using solution-based titanium oxide as an optical spacer. Adv Mater 18:572–576
Scharher MC, Muhlbacher D, Koppe M, Denk P, Waldauf C, Heeger AJ (2006) Design rules for donors in bulk-heterojunction solar cells toward 10 % energy-conversion efficiency. Adv Mater 18:789–794
Campaigne E, Budde WM, Schaefer GF (1963) p-Aminobenzaldehyde. Org Synth Coll 4:31
El-Shekeil AG, Al-Yusufy FA, Saknidy S (1997) DC conductivity of some polyazomethines. Polym Int 42:39–44
Dalelio GF, Feigi DM, Kieffer NE, Mehta RK (1968) Polymeric azomethines. J Macromol Sci Chem A2(6):1223–1234
Dalelio GF, Strazik WF, Feigl DM, Schoenig RK (1968) Polymeric Schiff base. Alternate synthesis for iminobenzylidene polymers. J Macromol Sci Chem A1:1457–1475
Hu L, Frech R, Glatzhofer DT, Mason R, York SS (2008) Linear poly(propylenimine)/lithium triflate as a polymer electrolyte system. Solid State Ion 179:401–408
Mason RN, Hu L, Glatzhofer DT, Frech R (2010) Infrared spectroscopic and conductivity studies of poly(N-methylpropylenimine)/lithium triflate electrolytes. Solid State Ion 180:1626–1632
Lopez-Garriga JJ, Babcock GT, Harrison JF (1986) Factors influencing the C:N stretching frequency in neutral and protonated Schiff’s base. J Am Chem Soc 108:7241–7251
Wang C, Shieh S, Legoff E, Kanatzidiz MG (1996) Synthesis and characterization of a new conjugated aromatic poly(azomethine) derivative based on the 3′,4′-dibutyl-α-terthiophene building block. Macromolecules 29:3147–3156
Colladet K, Nicolas M, Goris L, Lutsen L, Vanderzande D (2004) Low-band gap polymers for photovoltaic applications. Thin Solid Films 451(452):7–11
Acknowledgments
The authors thank Fondecyt 1120055 and DID-UACh (Grant No. S-2011-50) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, C.O., Huaiquimilla, D., Sobarzo, P. et al. Low band gap Schiff base polymers obtained by complexation with Lewis acids. Polym. Bull. 70, 971–984 (2013). https://doi.org/10.1007/s00289-012-0857-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-012-0857-0