Abstract
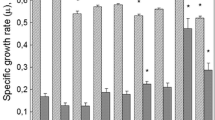
The role of SOD gene in response to UV-C radiations was studied in Pseudomonas aeruginosa. Firstly, our results showed that the inactivation of sodM and/or sodB genes decreases the resistance of P. aeruginosa after exposure to UV-C rays. Furthermore, our results showed that SOD activity is dose dependant in all strains. However, significant increase in SOD activity was only shown at UV-C exposure time of 5 min in sodB mutant. At an elevated dose equivalent to 30 min of exposure, significant increase in SOD activity was observed in sodM. Catalase activities showed significant decrease in WT and in sodB mutant after an exposure time of 30 min. CAT enzyme was present at higher levels than SOD, reflecting that alternate enzymes such as POX, is poorly associated with CAT activity, and an increase in POX activity is related to increase in stress tolerance. The overall results showed that sodB gene has an important protective role against UV-C radiations in P. aeruginosa, compared to SodM isoform.







Similar content being viewed by others
References
Blatchley ER (1996) Ultraviolet irradiation and chlorination/dechlorination for municipal wastewater disinfection. Water Environ Res 68:194–198
Meng JY, Zhang CY, Fen Z, Wang XP, Lei CL (2009) Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J Insect Physiol 55:588–592
Brooks E, Melnick JL, Adelberg EA (2007) Medical microbiology, vol 24. McGraw–Hill companies, New York
Wolfgang MC, Kulasekara BR, Liang X, Boyd D, Wu K, Yang Q, Miyada CG, Lory S (2003) Conservation of genome content and virulence determinants among clinical and environmental isolates of Pseudomonas aeruginosa. PNAS 100:8484–8489
Sahebjamei H, Abdolmaleki P, Ghanati F (2007) Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 28:42–47
Zhao G, Chen S, Wang L, Zhao Y, Wang J, Wang X, Zhang W, Wu R, Wu L, Wu Y, Xu A (2011) Cellular ATP content was decreased by a homogeneous 8.5 T static magnetic field exposure: role of reactive oxygen species. Bioelectromagnetics 32:94–101
Hassett DJ, Charniga L, Bean KA, Ohman DE, Cohen MS (1992) Antioxidant defense mechanisms in Pseudomonas aeruginosa: resistance to the redox-active antibiotic pyocyanin and demonstration of a manganese- cofactored superoxide dismutase. Infect Immun 60:328–336
Hassett DJ, Woodruff WA, Wozniak DJ, Vasil ML, Cohen MS, Ohman DE (1993) Cloning of the sodA and sodB genes encoding manganese and iron superoxide dismutase in Pseudomonas aeruginosa: demonstration of increased manganese superoxide dismutase activity in alginate-producing bacteria. J Bacteriol 175:7658–7665
Iiyama K, ChiedaY Lee JM, Kusakabe T, Yasunaga-Aoki C, Shimizu S (2007) Effect of superoxide dismutase gene inactivation on virulence of Pseudomonas aeruginosa PAO1 toward the silkworm, Bombyx mori. App Env Microbiol 73:1569–1575
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by Catalase. J Biol Chem 195:133–141
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Nikerson RG, Tworkoski TJ, Luster DG (1993) Colletotrichum coccodes and thidiazuron alter specific peroxidase activities in velvetleaf (Abutilon theophrasti). Physiol Mol Plant Pathol 43:47–56
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900
Hassett DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177:6330–6337
Yoon SJ, Park JE, Yang JH, Park JW (2002) OxyR regulon controls lipid peroxidation-mediated oxidative stress in Escherichia coli. Biochem Mol Biol 35:297–301
Pricewhelan A, Dietrich LEP, Newman DK (2006) Rethinking secondary metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2:71–78
Venkataraman A, Rosenbaum M, Arends jan BA, Halitschke R, Angenent LT (2010) Quorum sensing regulates electric current generations of Pseudomonas aeruginosa PA14 in bioelectrochemical systems. Electrochem Commun 12:459–462
Reszka KJ, OMalley Y, McCormick ML, Denning GM, Britigan BE (2004) Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa by microperoxidase 11 and hydrogen peroxide. Free Radic Biol Med 36:1448–1459
Elkins JG, Hassett DJ, Stewart PS, Schweizer HP, McDermott TR (1999) Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl Environ Microbiol 65:4594–4600
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbal, S.K.B., Maalej, L., Chourabi, K. et al. Antioxidant Defense Mechanisms in Pseudomonas aeruginosa: Role of Iron-Cofactored Superoxide Dismutase in Response to UV-C Radiations. Curr Microbiol 73, 159–164 (2016). https://doi.org/10.1007/s00284-016-1043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1043-7