Abstract
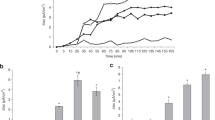
The pathogenic mechanism of Vibrio cholerae manifests as diarrhea and causes life-threatening dehydration. Here, we observe the human intestinal epithelial cells (HIEC) response to Cholera toxin (CT) by a real-time cell analysis (RTCA) platform, and disclose the difference from CT-induced cytotoxicity and others in HIEC. An HIEC cell of 1.0 × 105 cells/mL was characterized as the suitable concentration for each well. For experimentation, the assay requires an inoculation of CT dissolved in Dulbecco’s phosphate-buffered saline with 0.1 % gelatin for a period of 18–25 h. The dimensionless impedance cell index curve presented characteristic dose- and time-dependent drop responses at the first stage, and the CT-induced cytotoxicity was the most remarkable following exposure for 18–25 h (P = 0.0002). Following the obvious cytotoxic reaction, the CI curve gradually increased over time until the original CI value, indicating that self-recovery occurred. The CT-induced CI curve for HIEC was different from that induced by other toxins, including diphtheria and Clostridium difficile toxin. Collectively, these results suggest that the CT-induced cytotoxicity in HIEC was absolutely different from that induced by C. difficile and other toxins because of the different pathogeneses that were correlated with the specific CI curve generated by the RTCA system. In summary, our data show that the assay described here is a convenient and rapid high-throughput tool for real-time monitoring of host cellular responses to CT on the basis of the characteristic CI curve.





Similar content being viewed by others
References
Atienza JM, Zhu J, Wang X, Xu X, Abassi Y (2005) Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J Biomol Screen 10:795–805
Bernas T, Dobrucki J (2002) Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 47:236–242
Chinnapen DJ, Chinnapen H, Saslowsky D, Lencer WI (2007) Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol Lett 266:129–137
De Haan L, Hirst TR (2004) Cholera toxin: a paradigm for multi-functional engagement of cellular mechanisms (Review). Mol Membr Biol 21:77–92
Donta ST (1974) Differentiation between the steroidogenic effects of cholera enterotoxin and adrenocorticotropin through use of a mutant adrenal cell line. J Infect Dis 129:728–731
Guerrant RL, Brunton LL, Schnaitman TC, Rebhun LI, Gilman AG (1974) Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun 10:320–327
He X, Wang J, Steele J, Sun X, Nie W, Tzipori S, Feng H (2009) An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J Microbiol Methods 78:97–100
Holmes RK, Twiddy EM (1983) Characterization of monoclonal antibodies that react with unique and cross-reacting determinants of cholera enterotoxin and its subunits. Infect Immun 42:914–923
Holmgren J (1981) Actions of cholera toxin and the prevention and treatment of cholera. Nature 292:413–417
Irelan JT, Wu MJ, Morgan J, Ke N, Xi B, Wang X, Xu X, Abassi YA (2011) Rapid and quantitative assessment of cell quality, identity, and functionality for cell-based assays using real-time cellular analysis. J Biomol Screen 16:313–322
Jin D, Luo Y, Zheng M, Li H, Zhang J, Stampfl M, Xu X, Ding G, Zhang Y, Tang YW (2013) Quantitative detection of Vibrio cholera toxin by real-time and dynamic cytotoxicity monitoring. J Clin Microbiol 51:3968–3974
Kanwar JR, Kanwar RK (2009) Gut health immunomodulatory and anti-inflammatory functions of gut enzyme digested high protein micro-nutrient dietary supplement-Enprocal. BMC Immunol 10:7
Kaper JB, Morris JG Jr., Levine MM (1995) Cholera. Clin Microbiol Rev 8:48–86
Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P (2008) Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44:205–221
Krysko DV, Vanden Berghe T, Parthoens E, D’Herde K, Vandenabeele P (2008) Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Methods Enzymol 442:307–341
Lockshin RA, Zakeri Z (2004) Apoptosis, autophagy, and more. Int J Biochem Cell Biol 36:2405–2419
Martinez-Serra J, Gutierrez A, Munoz-Capo S, Navarro-Palou M, Ros T, Amat JC, Lopez B, Marcus TF, Fueyo L, Suquia AG, Gines J, Rubio F, Ramos R, Besalduch J (2014) xCELLigence system for real-time label-free monitoring of growth and viability of cell lines from hematological malignancies. Onco Targets Ther 7:985–994
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Moss J, Vaughan M (1979) Activation of adenylate cyclase by choleragen. Annu Rev Biochem 48:581–600
Ryder AB, Huang Y, Li H, Zheng M, Wang X, Stratton CW, Xu X, Tang YW (2010) Assessment of Clostridium difficile infections by quantitative detection of tcdB toxin by use of a real-time cell analysis system. J Clin Microbiol 48:4129–4134
Sears CL, Kaper JB (1996) Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev 60:167–215
Slanina H, Konig A, Claus H, Frosch M, Schubert-Unkmeir A (2011) Real-time impedance analysis of host cell response to meningococcal infection. J Microbiol Methods 84:101–108
Solly K, Wang X, Xu X, Strulovici B, Zheng W (2004) Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol 2:363–372
Spangler BD (1992) Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev 56:622–647
Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E, Modjtahedi N, Kroemer G (2008) Methods for assessing autophagy and autophagic cell death. Methods Mol Biol 445:29–76
Tian D, Zhang W, He J, Liu Y, Song Z, Zhou Z, Zheng M, Hu Y (2012) Novel, real-time cell analysis for measuring viral cytopathogenesis and the efficacy of neutralizing antibodies to the 2009 influenza A (H1N1) virus. PLoS One 7:e31965
Vanden Broeck D, Horvath C, De Wolf MJ (2007) Vibrio cholerae: cholera toxin. Int J Biochem Cell Biol 39:1771–1775
Witkowski PT, Schuenadel L, Wiethaus J, Bourquain DR, Kurth A, Nitsche A (2010) Cellular impedance measurement as a new tool for poxvirus titration, antibody neutralization testing and evaluation of antiviral substances. Biochem Biophys Res Commun 401:37–41
Xing JZ, Zhu L, Jackson JA, Gabos S, Sun XJ, Wang XB, Xu X (2005) Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem Res Toxicol 18:154–161
Yu N, Atienza JM, Bernard J, Blanc S, Zhu J, Wang X, Xu X, Abassi YA (2006) Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Anal Chem 78:35–43
Acknowledgments
This work was supported in part by the program for Zhejiang Leading Team of Science and Technology Innovation (2011R50021-21).
Author information
Authors and Affiliations
Corresponding author
Additional information
Julian Ye and Yun Luo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, J., Luo, Y., Fang, W. et al. Real-Time Cell Analysis for Monitoring Cholera Toxin-Induced Human Intestinal Epithelial Cell Response. Curr Microbiol 70, 536–543 (2015). https://doi.org/10.1007/s00284-014-0752-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0752-z