Abstract
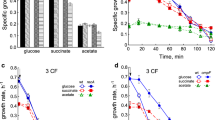
Effect of deletion of acid resistant genes of E. coli on the high-pressure carbon dioxide (HPC) resistance was investigated. Genes coding amino acid decarboxylases, such as lysine, arginine, and glutamate decarboxylase, were found to contribute to HPC resistance. Protonophore-treated cells showed hypersensitivity to HPC, confirming that HPC induced cytoplasm acidification and exerted severe damage on cells by intrusion of gaseous carbon dioxide into cytoplasm.


Similar content being viewed by others
References
Battesti A, Majdalani N, Gottesman S (2011) The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213
Castanie´-Cornet, MP, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535
Castanié-Cornet MP, Cam K, Bastiat B, Cros A, Bordes P, Gutierrez C (2010) Acid stress response in Escherichia coli: mechanism of regulation of gadA transcription by RcsB and GadE. Nucleic Acids Res 38:3546–3554
Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS (2010) ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:71–99
De Biase D, Tramonti A, Bossa F, Visca P (1999) The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol Microbiol 32:1198–1211
Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R (1999) Bacterial inactivation by using near- and supercritical carbon dioxide. Proc Natl Acad Sci USA. 96:10344–10348
Erkmen O (2001) Effect of high-pressure carbon dioxide on Escherichia coli in nutrient broth and milk. Int J Food Microbiol 65:131–135
Foster JW (2004) Escherichia coli acid resistance: tales of an amateur acidophile. Nature Rev Microbiol 2:898–907
Furukawa S, Watanabe T, Tai T, Hirata J, Ogihara H, Yamasaki M (2003) Effect of high pressure gaseous and supercritical carbon dioxide treatments on the bacterial spores. Biocontrol Sci 8:97–100
Furukawa S, Watanabe T, Tai T, Hirata J, Narisawa N, Kawarai T, Ogihara H, Yamasaki M (2004) Effect of high pressure gaseous carbon dioxide on the germination of bacterial spores. Int J Food Microbiol 91:209–213
Furukawa S, Watanabe T, Koyama T, Hiratal J, Narisawa N, Ogihara H, Yamasaki M (2009) Inactivation of food poisoning bacteria and Geobacillus stearothermophilus spores by high pressure carbon dioxide treatment. Food Control 20:53–58
Garcia-Gonzalez L, Geeraerd AH, Spilimbergo S, Elst K, Van Ginneken L, Debevere J, Van Impe JF, Devlieghere F (2007) High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int J Food Microbiol 117:1–28
Ishikawa H, Shimoda M, Shiratsuchi H, Osajima Y (1995) Sterilization of microorganisms by the supercritical carbon dioxide micro-bubble method. Biosci Biotechnol Biochem 59:1949–1950
Knorr D, Heinz V (2001) Development of nonthermal methods for microbial control. In: Block SS (ed) Disinfection, sterilization, and preservation. Lea & Febiger, London, pp 853–877
Lee JH, Lennon CW, Ross W, Gourse RL (2012) Role of the coiled-coil tip of Escherichia coli DksA in promoter control. J Mol Biol 416:503–517
Nakamura K, Enomoto A, Fukuhisa H, Nagai K, Hakoda M (1994) Disruption of microbial cells by the flash discharge of high-pressure carbon dioxide. Biosci Biotechnol Biochem 58:1297–1301
Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL (2004) DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118:311–322
Perederina A, Svetlov V, Vassylyeva MN, Tahirov TH, Yokoyama S, Artsimovitch I, Vassylyev DG (2004) Regulation through the secondary channel-structural framework for ppGpp-DksA synergism during transcription. Cell 118:297–309
Sharma UK, Chatterji D (2010) Transcriptional switching in Escherichia coli during stress and starvation by modulation of σ70 activity. FEMS Microbiol Rev 34:646–657
Shimoda M, Yamamoto Y, Cocunubo-Castellanos J, Tonoike H, Kawano T, Ishikawa H, Osajima Y (1998) Antimicrobial Effects of Pressured Carbon Dioxide in a Continuous Flow System. J Food Sci 63:709–712
Srivatsan A, Wang JD (2008) Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol 11:100–105
Watanabe T, Furukawa S, Hirata J, Koyama T, Ogihara H, Yamasaki M (2003) Inactivation of Geobacillus stearothermophilus spores by high-pressure carbon dioxide treatment. Appl Environ Microbiol 69:7124–7129
Watanabe T, Furukawa S, Tai T, Hirata J, Narisawa N, Ogihara H, Yamasaki M (2003) High pressure carbon dioxide decreases the heat tolerance of the bacterial spores. Food Sci. Technol. Res. 9:342–344
Watanabe T, Furukawa S, Kitamoto K, Takatsuki A, Hirata R, Ogihara H, Yamasaki M (2005) Vacuolar H+-ATPase and plasma membrane H+-ATPase contribute to the tolerance against high-pressure carbon dioxide treatment in Saccharomyces cerevisiae. Int J Food Microbiol 105:131–137
Watanabe T, Furukawa S, Kawarai T, Wachi M, Ogihara H, Yamasaki M (2007) Cytoplasmic acidification may occur in high-pressure carbon dioxide-treated Escherichia coli K12. Biosci Biotechnol Biochem 71:2522–2526
Yamamoto Y, Miwa Y, Miyoshi K, Furuyama J, Ohmori H (1997) The Escherichia coli ldcC gene encodes another lysine decarboxylase, probably a constitutive enzyme. Genes Genet Syst 72:167–172
Acknowledgments
We would like to thank the National Institute of Genetics (Shizuoka) and National BioResource Project (NIG, Japan): E. coli for giving us the E. coli strains and plasmids used in this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Furukawa, S., Shimazaki, J., Kawaharada, K. et al. Acid Resistance Contributes to the High-Pressure Carbon Dioxide Resistance of Escherichia coli K-12. Curr Microbiol 70, 1–5 (2015). https://doi.org/10.1007/s00284-014-0674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0674-9