Abstract
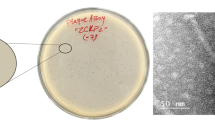
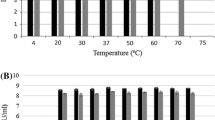
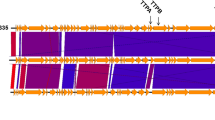
Klebsiella bacteria have emerged as an increasingly important cause of community-acquired nosocomial infections. Extensive use of broad-spectrum antibiotics in hospitalised patients has led to both increased carriage of Klebsiella and the development of multidrug-resistant strains that frequently produce extended-spectrum β-lactamases and/or other defences against antibiotics. Many of these strains are highly virulent and exhibit a strong propensity to spread. In this study, six lytic Klebsiella bacteriophages were isolated from sewage-contaminated river water in Georgia and characterised as phage therapy candidates. Two of the phages were investigated in greater detail. Biological properties, including phage morphology, nucleic acid composition, host range, growth phenotype, and thermal and pH stability were studied for all six phages. Limited sample sequencing was performed to define the phylogeny of the K. pneumoniae- and K. oxytoca-specific bacteriophages vB_Klp_5 and vB_Klox_2, respectively. Both of the latter phages had large burst sizes, efficient rates of adsorption and were stable under different adverse conditions. Phages reported in this study are double-stranded DNA bacterial viruses belonging to the families Podoviridae and Siphoviridae. One or more of the six phages was capable of efficiently lysing ~63 % of Klebsiella strains comprising a collection of 123 clinical isolates from Georgia and the United Kingdom. These phages exhibit a number of properties indicative of potential utility in phage therapy cocktails.





Similar content being viewed by others
References
Adams M (1959) Bacteriophages. Interscience, New York, pp 137–150
Drulis-Kawa Z, Mackiewicz P, Kesik-Szeloch A, Maciaszczyk-Dziubinska E, Weber-Dabrowska B, Dorotkiewicz-Jach A, Augustyniak D, Majkowska-Skrobek G, Bocer T, Empel J, Kropinski AM (2011) Isolation and characterisation of KP34-a novel phiKMV-like bacteriophage for Klebsiella pneumoniae. Appl Microbiol Biotechnol 90:1333–1345. doi:10.1007/s00253-011-3149-y
Gatedee J, Kritsiriwuthinan K, Galyov EE, Shan J, Dubinina E, Intarak N, Clokie MR, Korbsrisate S (2011) Isolation and characterization of a novel podovirus which infects Burkholderia pseudomallei. Virol J 8:366. doi:10.1186/1743-422X-8-366
Gu J, Liu X, Li Y, Han W, Lei L, Yang Y, Zhao H, Gao Y, Song J, Lu R, Sun C, Feng X (2012) A method for generation phage cocktail with great therapeutic potential. PLoS One 7:e31698. doi:10.1371/journal.pone.0031698
Hyman P, Abedon ST (2009) Practical methods for determining phage growth parameters. Methods Mol Biol 501:175–202. doi:10.1007/978-1-60327-164-6_18
Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP (2009) Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol 501:69–76. doi:10.1007/978-1-60327-164-6_7
Kropinski AM, Prangishvili D, Lavigne R (2009) Position paper: the creation of a rational scheme for the nomenclature of viruses of Bacteria and Archaea. Environ Microbiol 11:2775–2777. doi:10.1111/j.1462-2920.2009.01970.x
Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi:10.1016/S1473-3099(10)70143-2
Kumari S, Harjai K, Chhibber S (2010) Evidence to support the therapeutic potential of bacteriophage Kpn5 in burn wound infection caused by Klebsiella pneumoniae in BALB/c mice. J Microbiol Biotechnol 20:935–941
Kutter E (2009) Phage host range and efficiency of plating. Methods Mol Biol 501:141–149. doi:10.1007/978-1-60327-164-6_14
Lee M, Miller RC Jr (1974) T7 exonuclease (gene 6) is necessary for molecular recombination of bacteriophage T7. J Virol 14:1040–1048
Lingohr E, Frost S, Johnson RP (2009) Determination of bacteriophage genome size by pulsed-field gel electrophoresis. Methods Mol Biol 502:19–25. doi:10.1007/978-1-60327-565-1_3
Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD (2011) Molecular dissection of an outbreak of carbapenem-resistant enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2:e00204–e00211. doi:10.1128/mBio.00204-11
Nagaraj S, Chandran SP, Shamanna P, Macaden R (2012) Carbapenem resistance among Escherichia coli and Klebsiella pneumoniae in a tertiary care hospital in south India. Indian J Med Microbiol 30:93–95. doi:10.4103/0255-0857.93054
Nale JY, Shan J, Hickenbotham PT, Fawley WN, Wilcox MH, Clokie MR (2012) Diverse temperate bacteriophage carriage in Clostridium difficile 027 strains. PLoS One 7:e37263. doi:10.1371/journal.pone.0037263
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603
Ramphal R, Ambrose PG (2006) Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin Infect Dis 42(Suppl 4):S164–S172. doi:10.1086/500663
Serwer P, Watson RH, Son M (1990) Role of gene 6 exonuclease in the replication and packaging of bacteriophage T7 DNA. J Mol Biol 215:287–299. doi:10.1016/S0022-2836(05)80347-X
Son M, Serwer P (1992) Role of exonuclease in the specificity of bacteriophage T7 DNA packaging. Virology 190:824–833
Stahlhut SG, Struve C, Krogfelt KA, Reisner A (2012) Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol Med Microbiol 65:350–359. doi:10.1111/j.1574-695X.2012.00965.x
Sulakvelidze A, Kutter E (2005) Bacteriophage therapy in humans. In: Kutter E, Sulakvelidze A (eds) Bacteriophages: biology and applications. CRC, New York, pp 377–428
Sulakvelidze A, Alavidze Z, Morris JG Jr (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659. doi:10.1128/AAC.45.3.649-659.2001
Summers WC(1999) Felix d’Herelle and the origins of molecular biology. Yale University Press, New Haven, pp 108–124
Tsai SS, Huang JC, Chen ST, Sun JH, Wang CC, Lin SF, Hsu BR, Lin JD, Huang SY, Huang YY (2010) Characteristics of Klebsiella pneumoniae bacteremia in community-acquired and nosocomial infections in diabetic patients. Chang Gung Med J 33:532–539
Tsay RW, Siu LK, Fung CP, Chang FY (2002) Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med 162:1021–1027
Verma V, Harjai K, Chhibber S (2009) Restricting ciprofloxacin-induced resistant variant formation in biofilm of Klebsiella pneumoniae B5055 by complementary bacteriophage treatment. J Antimicrob Chemother 64:1212–1218. doi:10.1093/jac/dkp360
Acknowledgments
We thank Seydina M. Diene, PhD, Pathologie Humaine/Maladies infectieuses, Faculté de Médecine et de Pharmacie, Université de la Méditerranée Aix Marseille II, for help with bioinformatics analyses. This study was supported by a GNSF (Georgian National Science Foundation) grant N 04/01 to NK. Work in KR’s laboratory was partly funded by a Medisearch grant.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Karumidze, N., Kusradze, I., Rigvava, S. et al. Isolation and Characterisation of Lytic Bacteriophages of Klebsiella pneumoniae and Klebsiella oxytoca . Curr Microbiol 66, 251–258 (2013). https://doi.org/10.1007/s00284-012-0264-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0264-7