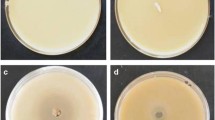
Abstract
Screening for cellulase-producing microorganisms is routinely done on carboxymethylcellulose (CMC) plates. The culture plates are flooded either with 1% hexadecyltrimethyl ammonium bromide or with 0.1% Congo red followed by 1 M NaCl. In both cases, it takes a minimum of 30 to 40 minutes to obtain the zone of hydrolysis after flooding, and the hydrolyzed area is not sharply discernible. An improved method is reported herein for the detection of extracellular cellulase production by microorganisms by way of plate assay. In this method, CMC plates were flooded with Gram’s iodine instead of the reagents just mentioned. Gram’s iodine formed a bluish-black complex with cellulose but not with hydrolyzed cellulose, giving a sharp and distinct zone around the cellulase-producing microbial colonies within 3 to 5 minutes. The new method is rapid and efficient; therefore, it can be easily performed for screening large numbers of microbial cultures of both bacteria and fungi. This is the first report on the use of Gram’s iodine for the detection of cellulase production by microorganisms using plate assay.





Similar content being viewed by others
References
Beguin P, Anbert JP (1993) The biological degradation of cellulose. FEMS Microbiol Rev 13:25–58
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Camassola M, Dillon AJP (2007) Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J Appl Microbiol 103:2196–2204
Coughlan MP (1985) The production of fungal and bacterial cellulases with comment on their production and application. Biotechnol Genet Eng Rev 13:39–109
Gusakov AV, Berlin AG, Popova NN, Okunev ON, Sinitsyn AO, Sinitsyn AP (2000) A comparative study of different cellulase preparations in the enzymatic treatment of cotton fabrics. Appl Biochem Biotechnol 88:119–126
Hankin L, Anagnostakis S (1977) Solid media containing carboxy methyl cellulose to detect CM cellulase activity of microorganisms. J Gen Microbiol 98:109–115
Hendricks CW, Doyle JD, Hugley B (1995) A new solid medium for enumerating cellulose-utilizing bacteria in soil. Appl Environ Microbiol 61:2016–2019
Ito S (1997) Alkaline cellulases from alkaliphilic Bacillus: Enzymatic properties, genetics and application to detergents. Extremophiles 1:61–66
Kasana RC, Sharma UK, Sharma N, Sinha AK (2007) Isolation and identification of a novel strain of Pseudomonas chlororaphis capable of transforming isoeugenol to vanillin. Curr Microbiol 54:457–461
Lamb J, Loy T (2005) Seeing red: The use of Congo Red dye to identify cooked and damaged starch grains in archaeological residues. J Archaeol Sci 32:1433–1440
Mandels M (1985) Applications of cellulases. Biochem Soc Trans 13:414–415
Park J, Park K (2001) Improvement of the physical properties of reprocessed paper by using biological treatment with modified cellulase. Bioresour Technol 79:91–94
Tanaka K, Mii T, Marui S, Matsubara I, Igaki H (1981) Mutagenicity of urinary metabolites of benzidine and benzidine-based azo dyes. Int Arch Occup Environ Health 49:177–185
Teather RM, Wood PJ (1982) Use of Congo red polysaccharide interactions complex formation between Congo red and polysaccharide in detection and assay of polysaccharide hydrolases. Methods Enzymol 160:59–74
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, NY, pp 315–322
Acknowledgments
The authors are grateful to P. S. Ahuja, Director, Institute of Himalayan Bioresource Technology (CSIR), Palampur, India, for providing the necessary laboratory facility. The Council of Scientific and Industrial Research (CSIR) is also acknowledged for providing financial support under the project NWP06. This is IHBT communication No. 0839.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasana, R.C., Salwan, R., Dhar, H. et al. A Rapid and Easy Method for the Detection of Microbial Cellulases on Agar Plates Using Gram’s Iodine. Curr Microbiol 57, 503–507 (2008). https://doi.org/10.1007/s00284-008-9276-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9276-8