Abstract
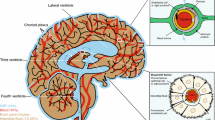
The brain is the organ with the highest metabolic demand in the body. Therefore, it needs specialized vasculature to provide it with the necessary oxygen and nutrients, while protecting it against pathogens and toxins. The blood-brain barrier (BBB) is very tightly regulated by specialized endothelial cells, two basement membranes, and astrocytic endfeet. The proximity of astrocytes to the vessel makes them perfect candidates to influence the function of the BBB. Moreover, other glial cells are also known to contribute to either BBB quiescence or breakdown. In this review, we summarize the knowledge on glial regulation of the BBB during development, in homeostatic conditions in the adult, and during neuroinflammatory responses.

Similar content being viewed by others
References
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harbor Perspect Biol 7(1). doi:10.1101/cshperspect.a020412
Alvarez JI, Katayama T, Prat A (2013) Glial influence on the blood brain barrier. Glia 61(12):1939–1958. doi:10.1002/glia.22575
Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1(5):409–417. doi:10.1002/ana.410010502
Coomber BL, Stewart PA (1985) Morphometric analysis of CNS microvascular endothelium. Microvasc Res 30(1):99–115
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. doi:10.1038/nrn1824
Alvarez JI, Cayrol R, Prat A (2011) Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 1812(2):252–264. doi:10.1016/j.bbadis.2010.06.017
Feeney JF Jr, Watterson RL (1946) The development of the vascular pattern within the walls of the central nervous system of the chick embryo. J Morphol 78:231–303
Bar T (1980) The vascular system of the cerebral cortex. Adv Anat Embryol Cell Biol 59:I-VI,1-62
Risau W, Wolburg H (1990) Development of the blood-brain barrier. Trends Neurosci 13(5):174–178
Norman MG, O'Kusky JR (1986) The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol 45(3):222–232
Ballabh P, Hu F, Kumarasiri M, Braun A, Nedergaard M (2005) Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr Res 58(4):791–798. doi:10.1203/01.PDR.0000180535.14093.FB
Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT (2002) Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16(20):2684–2698. doi:10.1101/gad.242002
Hagedorn M, Balke M, Schmidt A, Bloch W, Kurz H, Javerzat S, Rousseau B, Wilting J, Bikfalvi A (2004) VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev Dyn Off Publ Am Assoc Anatomists 230(1):23–33. doi:10.1002/dvdy.20020
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87(7):1171–1180
Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, Weimer RM, Carano RA, van Bruggen N, Watts RJ (2012) Death receptors DR6 and TROY regulate brain vascular development. Dev Cell 22(2):403–417. doi:10.1016/j.devcel.2011.11.018
Goldshmit Y, Galea MP, Bartlett PF, Turnley AM (2006) EphA4 regulates central nervous system vascular formation. J Comp Neurol 497(6):864–875. doi:10.1002/cne.21029
Tu T, Zhang C, Yan H, Luo Y, Kong R, Wen P, Ye Z, Chen J, Feng J, Liu F, Wu JY, Yan X (2015) CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. doi:10.1038/cr.2015.15
Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lecuyer MA, Ifergan I, Viel E, Bourbonniere L, Beauseigle D, Terouz S, Hachehouche L, Gendron S, Poirier J, Jobin C, Duquette P, Flanagan K, Yednock T, Arbour N, Prat A (2012) Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain J Neurol 135(Pt 10):2906–2924. doi:10.1093/brain/aws212
Moore SW, Tessier-Lavigne M, Kennedy TE (2007) Netrins and their receptors. Adv Exp Med Biol 621:17–31. doi:10.1007/978-0-387-76715-4_2
Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E (2008) Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183(3):409–417. doi:10.1083/jcb.200806024
Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA (2009) Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 106(2):641–646. doi:10.1073/pnas.0805165106
Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J (2009) Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139(2):285–298. doi:10.1016/j.cell.2009.07.047
Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ (2010) Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330(6006):985–989. doi:10.1126/science.1196554
Zhou Y, Nathans J (2014) Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell 31(2):248–256. doi:10.1016/j.devcel.2014.08.018
Engelhardt B, Liebner S (2014) Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res 355(3):687–699. doi:10.1007/s00441-014-1811-2
Stewart PA, Wiley MJ (1981) Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol 84(1):183–192
Hagan N, Ben-Zvi A (2014) The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis. Semin Cell Dev Biol. doi:10.1016/j.semcdb.2014.12.006
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509(7501):507–511. doi:10.1038/nature13324
Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J (2012) Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151(6):1332–1344. doi:10.1016/j.cell.2012.10.042
Sohet F, Lin C, Munji RN, Lee SY, Ruderisch N, Soung A, Arnold TD, Derugin N, Vexler ZS, Yen FT, Daneman R (2015) LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol. doi:10.1083/jcb.201410131
Miller FN, Sims DE (1986) Contractile elements in the regulation of macromolecular permeability. Fed Proc 45(2):84–88
Lindahl P, Johansson BR, Leveen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277(5323):242–245
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468(7323):562–566. doi:10.1038/nature09513
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood-brain barrier. Nature 468(7323):557–561. doi:10.1038/nature09522
Janzer RC, Raff MC (1987) Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325(6101):253–257. doi:10.1038/325253a0
Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2(4):287–293. doi:10.1038/35067582
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436(7048):193–200. doi:10.1038/nature03875
Kornyei Z, Gocza E, Ruhl R, Orsolits B, Voros E, Szabo B, Vagovits B, Madarasz E (2007) Astroglia-derived retinoic acid is a key factor in glia-induced neurogenesis. FASEB J Off Publ Fed Am Soc Exp Biol 21(10):2496–2509. doi:10.1096/fj.06-7756com
Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, de Vries HE, Reijerkerk A (2013) Retinoic acid induces blood-brain barrier development. J Neurosc Off J Soc Neurosci 33(4):1660–1671. doi:10.1523/JNEUROSCI.1338-12.2013
Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130(4):691–703. doi:10.1016/j.cell.2007.06.054
Ma S, Kwon HJ, Johng H, Zang K, Huang Z (2013) Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol 11(1):e1001469. doi:10.1371/journal.pbio.1001469
Alliot F, Godin I, Pessac B (1999) Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117(2):145–152
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845. doi:10.1126/science.1194637
Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116(5):829–840. doi:10.1182/blood-2009-12-257832
Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K (2001) Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev 36(2-3):258–264
Fournier AE, GrandPre T, Strittmatter SM (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409(6818):341–346. doi:10.1038/35053072
Nash M, Pribiag H, Fournier AE, Jacobson C (2009) Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol 40(3):224–235. doi:10.1007/s12035-009-8083-y
Aird WC (2012) Endothelial cell heterogeneity. Cold Spring Harbor Perspect Med 2(1):a006429. doi:10.1101/cshperspect.a006429
Daneman R (2012) The blood-brain barrier in health and disease. Ann Neurol 72(5):648–672. doi:10.1002/ana.23648
Girouard H, Iadecola C (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100(1):328–335. doi:10.1152/japplphysiol.00966.2005
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. doi:10.1016/j.neuron.2008.01.003
Rubin LL, Staddon JM (1999) The cell biology of the blood-brain barrier. Annu Rev Neurosci 22:11–28. doi:10.1146/annurev.neuro.22.1.11
Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH (2005) Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57(2):176–179. doi:10.1002/ana.20369
Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF (2007) Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology 68(21):1809–1814. doi:10.1212/01.wnl.0000262031.18018.1a
Sandoval KE, Witt KA (2008) Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32(2):200–219. doi:10.1016/j.nbd.2008.08.005
Jeynes B, Provias J (2011) The case for blood-brain barrier dysfunction in the pathogenesis of Alzheimer's disease. J Neurosci Res 89(1):22–28. doi:10.1002/jnr.22527
Cabezas R, Avila M, Gonzalez J, El-Bacha RS, Baez E, Garcia-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE (2014) Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci 8:211. doi:10.3389/fncel.2014.00211
Anderson JM, Van Itallie CM, Fanning AS (2004) Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16(2):140–145. doi:10.1016/j.ceb.2004.01.005
Anderson JM, Van Itallie CM (2009) Physiology and function of the tight junction. Cold Spring Harbor Perspect Biol 1(2):a002584. doi:10.1101/cshperspect.a002584
Knipp GT, Ho NF, Barsuhn CL, Borchardt RT (1997) Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci 86(10):1105–1110. doi:10.1021/js9700309
Furuse M, Sasaki H, Tsukita S (1999) Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147(4):891–903
Colegio OR, Van Itallie C, Rahner C, Anderson JM (2003) Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284(6):C1346–C1354. doi:10.1152/ajpcell.00547.2002
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. doi:10.1083/jcb.200302070
Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA (2010) The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5(10):e13741. doi:10.1371/journal.pone.0013741
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11(12):4131–4142
Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W (1999) A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci 112(Pt 12):1879–1888
Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL (1997) Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 110(Pt 14):1603–1613
Yeung D, Manias JL, Stewart DJ, Nag S (2008) Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol 115(6):635–642. doi:10.1007/s00401-008-0364-4
Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S (2004) Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem 279(43):44785–44794. doi:10.1074/jbc.M406563200
Lampugnani MG (2010) Endothelial adherens junctions and the actin cytoskeleton: an ‘infinity net’? J Biol 9(3):16. doi:10.1186/jbiol232
Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171(6):939–945. doi:10.1083/jcb.200510043
Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M (2011) LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci 124(Pt 4):548–555. doi:10.1242/jcs.072058
Loscher W, Potschka H (2005) Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx J Am Soc Exp NeuroTher 2(1):86–98. doi:10.1602/neurorx.2.1.86
Lee G, Bendayan R (2004) Functional expression and localization of P-glycoprotein in the central nervous system: relevance to the pathogenesis and treatment of neurological disorders. Pharm Res 21(8):1313–1330
Loscher W, Potschka H (2005) Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 76(1):22–76. doi:10.1016/j.pneurobio.2005.04.006
Thuerauf N, Fromm MF (2006) The role of the transporter P-glycoprotein for disposition and effects of centrally acting drugs and for the pathogenesis of CNS diseases. Eur Arch Psychiatry Clin Neurosci 256(5):281–286. doi:10.1007/s00406-006-0662-6
Cornford EM, Hyman S, Swartz BE (1994) The human brain GLUT1 glucose transporter: ultrastructural localization to the blood-brain barrier endothelia. J Cereb Blood Flow Metabol Off J Int Soc Cereb Blood Flow Metab 14(1):106–112. doi:10.1038/jcbfm.1994.15
Engelhardt B (2008) Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines. J Neurol Sci 274(1-2):23–26. doi:10.1016/j.jns.2008.05.019
Larochelle C, Alvarez JI, Prat A (2011) How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett 585(23):3770–3780. doi:10.1016/j.febslet.2011.04.066
Fries JW, Williams AJ, Atkins RC, Newman W, Lipscomb MF, Collins T (1993) Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol 143(3):725–737
Henninger DD, Panes J, Eppihimer M, Russell J, Gerritsen M, Anderson DC, Granger DN (1997) Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol 158(4):1825–1832
Engelhardt B, Ransohoff RM (2012) Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol 33(12):579–589. doi:10.1016/j.it.2012.07.004
Prat A, Biernacki K, Wosik K, Antel JP (2001) Glial cell influence on the human blood-brain barrier. Glia 36(2):145–155
Abbott NJ (2005) Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25(1):5–23
Pfaff D, Fiedler U, Augustin HG (2006) Emerging roles of the angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol 80(4):719–726. doi:10.1189/jlb.1105652
Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR (2009) VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A 106(6):1977–1982. doi:10.1073/pnas.0808698106
Shen F, Walker EJ, Jiang L, Degos V, Li J, Sun B, Heriyanto F, Young WL, Su H (2011) Coexpression of angiopoietin-1 with VEGF increases the structural integrity of the blood-brain barrier and reduces atrophy volume. J Cereb Blood Flow Metabol Off J Int Soc Cereb Blood Flow Metab 31(12):2343–2351. doi:10.1038/jcbfm.2011.97
Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW (2003) SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med 9(7):900–906. doi:10.1038/nm889
Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M (2008) The FGF system has a key role in regulating vascular integrity. J Clin Invest 118(10):3355–3366. doi:10.1172/JCI35298
Reuss B, Dono R, Unsicker K (2003) Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood-brain barrier permeability: evidence from mouse mutants. J Neurosci Off J Soc Neurosci 23(16):6404–6412
Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N (1999) Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophys Res Commun 261(1):108–112. doi:10.1006/bbrc.1999.0992
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A (2011) The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334(6063):1727–1731. doi:10.1126/science.1206936
Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342(18):1350–1358. doi:10.1056/NEJM200005043421807
Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y (2004) Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol 24(3):491–497
Baello S, Iqbal M, Bloise E, Javam M, Gibb W, Matthews SG (2014) TGF-beta1 regulation of multidrug resistance P-glycoprotein in the developing male blood-brain barrier. Endocrinology 155(2):475–484. doi:10.1210/en.2013-1472
Fabry Z, Topham DJ, Fee D, Herlein J, Carlino JA, Hart MN, Sriram S (1995) TGF-beta 2 decreases migration of lymphocytes in vitro and homing of cells into the central nervous system in vivo. J Immunol 155(1):325–332
Heinemann U, Kaufer D, Friedman A (2012) Blood-brain barrier dysfunction, TGFbeta signaling, and astrocyte dysfunction in epilepsy. Glia 60(8):1251–1257. doi:10.1002/glia.22311
Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A (2007) Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci Off J Soc Neurosci 27(34):9032–9042. doi:10.1523/JNEUROSCI.2088-07.2007
Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci Off J Soc Neurosci 31(45):16064–16069. doi:10.1523/JNEUROSCI.4158-11.2011
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131(6):1164–1178. doi:10.1016/j.cell.2007.10.036
Bialas AR, Stevens B (2013) TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16(12):1773–1782. doi:10.1038/nn.3560
Hughes V (2012) Microglia: the constant gardeners. Nature 485(7400):570–572. doi:10.1038/485570a
Schwartz M, Kipnis J, Rivest S, Prat A (2013) How do immune cells support and shape the brain in health, disease, and aging? J Neurosci Off J Soc Neurosci 33(45):17587–17596. doi:10.1523/JNEUROSCI.3241-13.2013
Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F, Taguchi A, Matsuyama T, Ihara M, Kim KW, Lo EH, Arai K (2014) Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One 9(7):e103174. doi:10.1371/journal.pone.0103174
Leong SY, Rao VT, Bin JM, Gris P, Sangaralingam M, Kennedy TE, Antel JP (2014) Heterogeneity of oligodendrocyte progenitor cells in adult human brain. Ann Clin transl Neurol 1(4):272–283. doi:10.1002/acn3.55
Eddleston M, Mucke L (1993) Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience 54(1):15–36
Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2(12):679–689. doi:10.1038/ncpneuro0355
Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81(2):229–248. doi:10.1016/j.neuron.2013.12.034
Gray F, Lescs MC, Keohane C, Paraire F, Marc B, Durigon M, Gherardi R (1992) Early brain changes in HIV infection: neuropathological study of 11 HIV seropositive, non-AIDS cases. J Neuropathol Exp Neurol 51(2):177–185
Girgrah N, Letarte M, Becker LE, Cruz TF, Theriault E, Moscarello MA (1991) Localization of the CD44 glycoprotein to fibrous astrocytes in normal white matter and to reactive astrocytes in active lesions in multiple sclerosis. J Neuropathol Exp Neurol 50(6):779–792
Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ (2000) Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol 161(1):102–114. doi:10.1006/exnr.1999.7269
Petito CK, Morgello S, Felix JC, Lesser ML (1990) The two patterns of reactive astrocytosis in postischemic rat brain. J Cereb Blood Flow Metabol Off J Int Soc Cereb Blood Flow Metab 10(6):850–859. doi:10.1038/jcbfm.1990.141
Jorgensen OS, Brooksbank BW, Balazs R (1990) Neuronal plasticity and astrocytic reaction in Down syndrome and Alzheimer disease. J Neurol Sci 98(1):63–79
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647. doi:10.1016/j.tins.2009.08.002
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. doi:10.1007/s00401-009-0619-8
Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M (2013) Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16(5):580–586. doi:10.1038/nn.3371
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23(2):297–308
Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D (2008) Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol 181(4):2683–2693
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci Off J Soc Neurosci 24(9):2143–2155. doi:10.1523/JNEUROSCI.3547-03.2004
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci Off J Soc Neurosci 29(37):11511–11522. doi:10.1523/JNEUROSCI.1514-09.2009
Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16(3):675–686
Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202(1):145–156. doi:10.1084/jem.20041918
Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR (2009) Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol 182(5):2628–2640. doi:10.4049/jimmunol.0802954
Gimsa U, Mitchison NA, Brunner-Weinzierl MC (2013) Immune privilege as an intrinsic CNS property: astrocytes protect the CNS against T-cell-mediated neuroinflammation. Mediat Inflamm 2013:320519. doi:10.1155/2013/320519
Koyama Y, Michinaga S (2012) Regulations of astrocytic functions by endothelins: roles in the pathophysiological responses of damaged brains. J Pharmacol Sci 118(4):401–407
Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, Prat A (2008) The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain J Neurol 131(Pt 3):785–799. doi:10.1093/brain/awm295
Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR (2014) Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62(3):452–467. doi:10.1002/glia.22616
Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122(4):1180–1188. doi:10.1172/JCI58649
International Multiple Sclerosis Genetics C, Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357(9):851–862. doi:10.1056/NEJMoa073493
Broux B, Hellings N, Venken K, Rummens JL, Hensen K, Van Wijmeersch B, Stinissen P (2010) Haplotype 4 of the multiple sclerosis-associated interleukin-7 receptor alpha gene influences the frequency of recent thymic emigrants. Genes Immun 11(4):326–333. doi:10.1038/gene.2009.106
Brosnan CF, Raine CS (2013) The astrocyte in multiple sclerosis revisited. Glia 61(4):453–465. doi:10.1002/glia.22443
Broux B, Stinissen P, Hellings N (2013) Which immune cells matter? The immunopathogenesis of multiple sclerosis. Crit Rev Immunol 33(4):283–306
Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A (2008) Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol 9(2):137–145. doi:10.1038/ni1551
Ifergan I, Kebir H, Alvarez JI, Marceau G, Bernard M, Bourbonniere L, Poirier J, Duquette P, Talbot PJ, Arbour N, Prat A (2011) Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain J Neurol 134(Pt 12):3560–3577. doi:10.1093/brain/awr268
Ifergan I, Kebir H, Terouz S, Alvarez JI, Lecuyer MA, Gendron S, Bourbonniere L, Dunay IR, Bouthillier A, Moumdjian R, Fontana A, Haqqani A, Klopstein A, Prinz M, Lopez-Vales R, Birchler T, Prat A (2011) Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol 70(5):751–763. doi:10.1002/ana.22519
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175. doi:10.1038/nm1651
Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, Bourbonniere L, Larochelle C, Prat A (2014) Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis 74C:14–24. doi:10.1016/j.nbd.2014.09.016
Luo J, Ho P, Steinman L, Wyss-Coray T (2008) Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J Neuroinflammation 5:6. doi:10.1186/1742-2094-5-6
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36(2):180–190
Chen Y, Swanson RA (2003) Astrocytes and brain injury. J Cereb Blood Flow Metabol Off J Int Soc Cereb Blood Flow Metab 23(2):137–149
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28(3):138–145. doi:10.1016/j.it.2007.01.005
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci CMLS 65(17):2702–2720. doi:10.1007/s00018-008-8059-5
Proescholdt MA, Jacobson S, Tresser N, Oldfield EH, Merrill MJ (2002) Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol 61(10):914–925
Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR (2006) IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol 177(8):5574–5584
Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR (2012) Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122(7):2454–2468. doi:10.1172/JCI60842
Shrestha B, Ge S, Pachter JS (2014) Resolution of central nervous system astrocytic and endothelial sources of CCL2 gene expression during evolving neuroinflammation. Fluids Barriers CNS 11(1):6. doi:10.1186/2045-8118-11-6
Paul D, Ge S, Lemire Y, Jellison ER, Serwanski DR, Ruddle NH, Pachter JS (2014) Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J Neuroinflammation 11:10. doi:10.1186/1742-2094-11-10
Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, Voskuhl RR (2014) Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol 274(1-2):53–61. doi:10.1016/j.jneuroim.2014.06.009
Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D (2014) Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci Off J Soc Neurosci 34(24):8175–8185. doi:10.1523/JNEUROSCI.1137-14.2014
Prat A, Biernacki K, Lavoie JF, Poirier J, Duquette P, Antel JP (2002) Migration of multiple sclerosis lymphocytes through brain endothelium. Arch Neurol 59(3):391–397
Ifergan I, Wosik K, Cayrol R, Kebir H, Auger C, Bernard M, Bouthillier A, Moumdjian R, Duquette P, Prat A (2006) Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol 60(1):45–55. doi:10.1002/ana.20875
Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, van Het Hof B, Scheffer G, Scheper R, Dijkstra CD, van der Valk P, de Vries HE (2011) Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain J Neurol 134(Pt 2):555–570. doi:10.1093/brain/awq330
Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K, Ellezam B, Bakshi R, Prat A, Antel JP, Weiner HL, Quintana FJ (2014) Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 20(10):1147–1156. doi:10.1038/nm.3681
Wang Y, Imitola J, Rasmussen S, O'Connor KC, Khoury SJ (2008) Paradoxical dysregulation of the neural stem cell pathway Sonic Hedgehog-Gli1 in autoimmune encephalomyelitis and multiple sclerosis. Ann Neurol 64(4):417–427. doi:10.1002/ana.21457
Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A (2014) Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic Hedgehog in astrocytes. PLoS One 9(10):e110024. doi:10.1371/journal.pone.0110024
Podjaski C, Alvarez JI, Bourbonniere L, Larouche S, Terouz S, Bin JM, Lécuyer M-A, Saint-Laurent O, Larochelle C, Darlington PJ, Arbour N, Antel JP, Kennedy TE, Prat A (2015) Netrin 1 regulates blood–brain barrier function and neuroinflammation. Brain 138(6):1598–612. doi:10.1093/brain/awv092
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57(6):563–581
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318. doi:10.1126/science.1110647
da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR (2014) The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 8:362. doi:10.3389/fncel.2014.00362
Rojo AI, McBean G, Cindric M, Egea J, Lopez MG, Rada P, Zarkovic N, Cuadrado A (2014) Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal 21(12):1766–1801. doi:10.1089/ars.2013.5745
Sumi N, Nishioku T, Takata F, Matsumoto J, Watanabe T, Shuto H, Yamauchi A, Dohgu S, Kataoka Y (2010) Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol Neurobiol 30(2):247–253. doi:10.1007/s10571-009-9446-7
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG (2006) Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke J Cereb Circ 37(4):1087–1093. doi:10.1161/01.STR.0000206281.77178.ac
Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y (2010) Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci 112(2):251–254
Bogie JF, Stinissen P, Hendriks JJ (2014) Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol 128(2):191–213. doi:10.1007/s00401-014-1310-2
van Horssen J, Singh S, van der Pol S, Kipp M, Lim JL, Peferoen L, Gerritsen W, Kooi EJ, Witte ME, Geurts JJ, de Vries HE, Peferoen-Baert R, van den Elsen PJ, van der Valk P, Amor S (2012) Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J Neuroinflammation 9:156. doi:10.1186/1742-2094-9-156
Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K (2012) Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3:1227. doi:10.1038/ncomms2230
Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H (2012) NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain J Neurol 135(Pt 3):886–899. doi:10.1093/brain/aws012
Rochfort KD, Collins LE, Murphy RP, Cummins PM (2014) Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One 9(7):e101815. doi:10.1371/journal.pone.0101815
Liu Y, Hao W, Letiembre M, Walter S, Kulanga M, Neumann H, Fassbender K (2006) Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci Off J Soc Neurosci 26(50):12904–12913. doi:10.1523/JNEUROSCI.2531-06.2006
Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD (2006) Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain J Neurol 129(Pt 2):517–526. doi:10.1093/brain/awh707
Bogie JF, Stinissen P, Hellings N, Hendriks JJ (2011) Myelin-phagocytosing macrophages modulate autoreactive T cell proliferation. J Neuroinflammation 8:85. doi:10.1186/1742-2094-8-85
Kotter MR, Li WW, Zhao C, Franklin RJ (2006) Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci Off J Soc Neurosci 26(1):328–332. doi:10.1523/JNEUROSCI.2615-05.2006
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211(8):1533–1549. doi:10.1084/jem.20132477
Acknowledgments
Dr. Prat is a senior Scholar of the FRQS. Dr. Broux is a postdoctoral fellow of Fonds voor Wetenschappelijk Onderzoek (FWO) Flanders. Ms. Gowing is funded by the Canadian Institutes of Health Research Strategic Training Program in Neuroinflammation. The work herein was funded by an operating grant from the MS Society of Canada.
The authors thank Phil Penalosa and Jérémie Lemarbre from Let There Be for their help in preparing the figure for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on : Role of Astrocytes and Microglia in CNS Inflammation - Guest Editor: Francisco Quintana
Rights and permissions
About this article
Cite this article
Broux, B., Gowing, E. & Prat, A. Glial regulation of the blood-brain barrier in health and disease. Semin Immunopathol 37, 577–590 (2015). https://doi.org/10.1007/s00281-015-0516-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0516-2