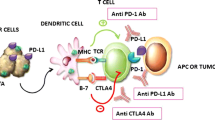
Abstract
Several studies suggest that the progression of malignant tumors as well as the response to chemotherapy and targeted therapy is critically dependent on the immunological parameters that are derived from the host immune system as well as a modulation of the immune system by therapeutic antibodies. It has been shown for many tumor types that the presence of a lymphocytic infiltrate in different types of cancers is a positive factor for clinical outcome and that the response to neoadjuvant chemotherapy is increased in a tumor with a prominent pretherapeutic infiltrate. Furthermore, new targeted therapies in breast cancer, such as trastuzumab, as well as in hematological malignancies, such as rituximab and alemtuzumab, have been shown to interact with immunological pathways, and this interaction is critical for response and clinical outcome. In neoplasms of lymphoid and hematopoietic tissues, targeted therapies not only reduce toxic effects on normal tissues but also lead to modulations of the immune system depending on the target molecule, its physiological function and cellular distribution. This review gives an overview on clinical data on response to classical chemotherapy as well as molecular targeted therapy and its interaction with the immune system.



Similar content being viewed by others
References
Nielsen DL, Andersson M, Kamby C (2009) HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat Rev 35:121–136
Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A (2010) Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol 21:815–819
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK (2010) ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2010) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Pohlmann PR, Mayer IA, Mernaugh R (2009) Resistance to trastuzumab in breast cancer. Clin Cancer Res 15:7479–7491
Kute TE, Savage L, Stehle JR Jr, Kim-Shapiro JW, Blanks MJ, Wood J, Vaughn JP (2009) Breast tumor cells isolated from in vitro resistance to trastuzumab remain sensitive to trastuzumab anti-tumor effects in vivo and to ADCC killing. Cancer Immunol Immunother 58:1887–1896
Countouriotis A, Moore TB, Sakamoto KM (2002) Cell surface antigen and molecular targeting in the treatment of hematologic malignancies. Stem Cells 20:215–229
Pagès F, Berger A, Camus M et al (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
DeNardo DG, Coussens LM (2007) Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 9:212
Aaltomaa S, Lipponen P, Eskelinen M et al (1992) Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer 28:859–864
Schmidt M, Böhm D, von Törne C et al (2008) The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 68:5405–5413
Zitvogel L, Kroemer G (2008) The immune response against dying tumor cells: avoid disaster, achieve cure. Cell Death Differ 15:1–2
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 220:47–59
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L (2008) Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 68:4026–4030
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8:59–73
Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G (2008) The anticancer immune response: indispensable for therapeutic success? J Clin Invest 118:1991–2001
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778–785
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113
Ménard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F (2008) Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother 57:1579–1587
Gianni L, Zambetti M, Clark K et al (2005) Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol 23:7265–7277
Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A (2008) Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest 26:1024–1031
Ladoire S, Arnould L, Apetoh L et al (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 14:2413–2420
Bates GJ, Fox SB, Han C et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Perez SA, Karamouzis MV, Skarlos DV et al (2007) CD4 + CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res 13:2714–2721
Fulton A, Miller F, Weise A, Wei WZ (2006–2007) Prospects of controlling breast cancer metastasis by immune intervention. Breast Dis 26:115–27
Beyer M, Schultze JL (2006) Regulatory T cells in cancer. Blood 108:804–811
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61:4766–4772
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 67:257–262
Murri AM, Hilmy M, Bell J, Wilson C, McNicol AM, Lannigan A, Doughty JC, McMillan DC (2008) The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer 99:1013–1019
Lamb GW, McArdle PA, Ramsey S, McNichol AM, Edwards J, Aitchison M, McMillan DC (2008) The relationship between the local and systemic inflammatory responses and survival in patients undergoing resection for localized renal cancer. BJU Int 102:756–761
Hilmy M, Campbell R, Bartlett JM, McNicol AM, Underwood MA, McMillan DC (2006) The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer 95:1234–1238
Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS (2005) The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 92:651–654
Iannello A, Ahmad A (2005) Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev 24:487–499
Ferris RL, Jaffee EM, Ferrone S (2010) Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol 28:4390–4399
Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA (2001) Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol 31:3016–3025
Parihar R, Dierksheide J, Hu Y, Carson WE (2002) IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest 110:983–992
Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A (2004) Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res 10:5650–5655
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 6:443–446
Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A (2008) Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 26:1789–1796
Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Ménard S, Costa A, Fagnoni FF (2007) Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res 67:11991–11999
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX (2011) Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat (in press)
Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L (2008) Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med 6:25
Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE (2006) Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol 13:1085–1098
zum Büschenfelde CM, Hermann C, Schmidt B, Peschel C, Bernhard H (2002) Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res 62:7
Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, Vahdat L, Cheng B, Pegram M, Knutson KL, Clynes R (2007) Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res 13:5133–5143
Milano F, Guarriera M, Rygiel AM, Krishnadath KK (2010) Trastuzumab mediated T-cell response against HER-2/neu overexpressing esophageal adenocarcinoma depends on intact antigen processing machinery. PLoS ONE 5:e12424
Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, Huang LQ, Murata S, Sgouros G, Emens LA, Reilly RT, Jaffee EM (2008) Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest 118:1700–1711
Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE 3rd (2006) Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res 66:517–526
Ferlazzo G, Münz C (2009) Dendritic cell interactions with NK cells from different tissues. J Clin Immunol 29:265–273
Whiteside TL, Stanson J, Shurin MR, Ferrone S (2004) Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol 173:1526–1534
Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J (2009) The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+T-cell axis in patients with breast cancer. Br J Cancer 100:1061–1067
Geary CG (2000) The story of chronic myeloid leukaemia. Br J Haematol 110:2–11
Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM (1999) The biology of chronic myeloid leukemia. N Engl J Med 341:164–172
Faderl S, Talpaz M, Estrov Z, Kantarjian HM (1999) Chronic myelogenous leukemia: biology and therapy. Ann Intern Med 131:207–219
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2:561–566
Deininger MW, Goldman JM, Lydon N, Melo JV (1997) The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood 90:3691–3698
Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J et al (2007) Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 110:4055–4063
Rohon P, Porkka K, Mustjoki S (2010) Immunoprofiling of patients with chronic myeloid leukemia at diagnosis and during tyrosine kinase inhibitor therapy. Eur J Haematol 85:387–398
Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP (2008) Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 111:1366–1377
Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Hoglund M, Kovanen P, Laurinolli T et al (2009) Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 23:1398–1405
Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Bohm A, Herndlhofer S, Tschachler E, Superti-Furga G, Jager U, Valent P (2009) Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest 39:1098–1109
Palanichamy A, Roll P, Theiss R, Dorner T, Tony HP (2008) Modulation of molecular imprints in the antigen-experienced B cell repertoire by rituximab. Arthritis Rheum 58:3665–3674
Eisenberg R (2005) Do autoantigens define autoimmunity or vice versa? Eur J Immunol 35:367–370
Kheirallah S, Caron P, Gross E, Quillet-Mary A, Bertrand-Michel J, Fournie JJ, Laurent G, Bezombes C (2010) Rituximab inhibits B-cell receptor signaling. Blood 115:985–994
Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH (2009) Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 182:5982–5993
Hale G (2001) The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy 3:137–143
Xia MQ, Hale G, Lifely MR, Ferguson MA, Campbell D, Packman L, Waldmann H (1993) Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J 293:633–640
Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Dyer MJ, Catovsky D (1998) Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res 22:185–191
Elsner J, Hochstetter R, Spiekermann K, Kapp A (1996) Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood 88:4684–4693
Gilleece MH, Dexter TM (1993) Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood 82:807–812
Rodig SJ, Abramson JS, Pinkus GS, Treon SP, Dorfman DM, Dong HY, Shipp MA, Kutok JL (2006) Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H). Clin Cancer Res 12:7174–7179
Rowan WC, Hale G, Tite JP, Brett SJ (1995) Cross-linking of the CAMPATH-1 antigen (CD52) triggers activation of normal human T lymphocytes. Int Immunol 7:69–77
Gribben JG, Hallek M (2009) Rediscovering alemtuzumab: current and emerging therapeutic roles. Br J Haematol 144:818–831
Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, Seaman S, Miller DH, Hale G, Waldmann H, Compston DA (2006) The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 253:98–108
Zent CS, Secreto CR, LaPlant BR, Bone ND, Call TG, Shanafelt TD, Jelinek DF, Tschumper RC, Kay NE (2008) Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res 32:1849–1856
Mone AP, Cheney C, Banks AL, Tridandapani S, Mehter N, Guster S, Lin T, Eisenbeis CF, Young DC, Byrd JC (2006) Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia 20:272–279
Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, Siders WM, Kaplan JM (2009) Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 128:260–270
Acknowledgment
We thank Martina Eickmann for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Prognostic Impact of Anti-Cancer Immune Responses
Rights and permissions
About this article
Cite this article
Denkert, C., Darb-Esfahani, S., Loibl, S. et al. Anti-cancer immune response mechanisms in neoadjuvant and targeted therapy. Semin Immunopathol 33, 341–351 (2011). https://doi.org/10.1007/s00281-011-0261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-011-0261-0