Abstract
Purpose
Codrituzumab, a humanized antibody against glypican-3, is highly expressed in HCC. A phase I study evaluated the combination with sorafenib in HCC.
Patients and methods
In a 3 + 3 design, codrituzumab was given intravenously in various doses with sorafenib 400 mg twice daily to patients with advanced HCC, age ≥18, ECOG 0–1, Child-Pugh A and B7, adequate organ functions, and no prior systemic therapy, with tumor assessment by RECIST 1.0 and safety by CTCAE 3.0. PK and pre, during, and post-therapy 124I radiolabeled codrituzumab PET scan imaging were performed.
Results
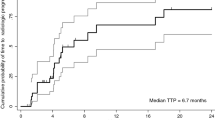
41 patients were enrolled: 2.5 mg/kg weekly (qw) (12), 5 mg/kg qw (12), 10 mg/kg qw (3), 1600 mg every 2 weeks (q2w) (6), and 1600 mg qw (7). Two drug limiting toxicities occurred: grade 3 hyponatremia at 5 mg/kg and grade 3 hyponatremia and hyperglycemia at 1600 mg q2w. Adverse events occurred in 80% of patients, including at least one ≥grade 3: ten (25%) increased AST, three (7.5%) increased ALT, and ten (25%) increased lipase. There were no responses and nine (25.7%) had stable disease. PK C max and AUCt of codrituzumab and sorafenib were comparable to single-agent data. Thirteen out of 14 patients showed 124I radiolabeled codrituzumab uptake in tumor. In all three patients who underwent a post-progression PET, glypican-3 remained expressed.
Conclusion
Codrituzumab plus sorafenib were tolerated at 1600 mg q2w and 400 mg bid, respectively, with no responses. Codrituzumab exerts selective distribution to HCC cells, and GPC3 does not show any down-regulation post-progression (NCT00976170).



Similar content being viewed by others
References
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34
Abou-Alfa GK, Venook AP (2013) The antiangiogenic ceiling in hepatocellular carcinoma: does it exist and has it been reached? Lancet Oncol 14(7):e283–e288
Filmus J, Selleck SB (2001) Glypicans: proteoglycans with a surprise. J Clin Invest 108:497–501
Yorita K, Takahashi N, Takai H, Kato A, Suzuki M, Ishiguro T et al (2011) Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int 31(1):120–131
Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M et al (2009) Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun 378(2):279–284
Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N et al (2013) First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 19(4):920–928
Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S, Kobayashi S, Morizane C et al (2014) Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci 105(4):455–462
Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H et al (2008) Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res 68(23):9832–8
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines). J Natl Cancer Inst 92:205–216
Abou-Alfa GK, Niedzwieski D, Knox JJ, Kaubisch A, Posey J, Tan BR, Kavan P, Goel R, Lammers PE, Bekaii-Saab TS, Tam VC, Rajdev L, Kelley RT, Siegel SB, Balletti J, Harding JJ, Schwartz LH, Goldberg RM, Bertagnolli MM, Venook AP (2016) Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol 34, (suppl; abstr 4003)
Chen YC et al (2014) Application of non-linear popPK with target mediated drug disposition (TMDD) in optimization of an oncology dosing regimen. ASCPT 2014 late breaking abstract, March 18–22
Shirakawa H, Suzuki H, Shimomura M, Kojima M et al (2009) Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 100(8):1403–1407
Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW et al (2016) Randomized Phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol 65(2):289–95.
Acknowledgements
We would like to acknowledge Amabella Lindo and Louise Harris for their expert nursing care, Jing Qiao and Ariel Brown for expert technical assistance in labeling the antibodies, Yiauchung Sheh for production of 124I, and Jiong (Lilly) Wu for handling of pharmacokinetic samples and assays and the radiopharmacy group for dispensing of the radiolabeled antibody. Pathologists, Kevin S. McDorman (Charles River Laboratories Inc., USA), Vikram Deshpande (Massachusetts General Hospital, USA), and Hiroaki Kataoka (University of Miyazaki, Japan) for pathological evaluations of GPC3-IHC, Monica Reinholz for technical assistance (Ventana Medical Systems Inc.), Ikue Suzuki, Sylvia Chiu, and Zhaodi Gu for their assistance. Funding was supported by Chugai Pharmaceutical Co. Ltd. and the Hoffmann-La Roche Inc. The Memorial Sloan Kettering Cancer Center’s Radiochemistry and Molecular Imaging Probes were supported by the radiochemistry core NIH grant P30CA008748) and the Ludwig Center for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ghassan K. Abou-Alfa, Chia-Jui Yen, Catherine Frenette, Bert O’Neil, Steven M. Larson, Ann-Lii Cheng, and Jorge A. Carrasquilo report research funding support from Roche and Chugai tot heir institutions. Toshihiko Ohtomo, Takayoshi Tanaka, Hideo Morikawa, Yuko Maki, and Norihisa Ohishi report Chugai employment. Ya-Chi Chen, Tamara Agajanov, Frederic Boisserie, Laura Di Laurenzio, and Ray Lee report Roche employment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abou-Alfa, G.K., Yen, CJ., Hsu, CH. et al. Phase Ib study of codrituzumab in combination with sorafenib in patients with non-curable advanced hepatocellular carcinoma (HCC). Cancer Chemother Pharmacol 79, 421–429 (2017). https://doi.org/10.1007/s00280-017-3241-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3241-9