Abstract
Background
We evaluated the efficacy and safety of 5-weekly S-1 and cisplatin combined with trastuzumab, a monoclonal antibody against human epidermal growth factor receptor type 2 (HER2) for HER2-positive advanced gastric cancer (AGC).
Methods
This phase II study treatment consisted of S-1 (80–120 mg per day) orally on day 1–21, cisplatin (60 mg/m2) intravenously on day 8, and trastuzumab (8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg every 3 weeks) intravenously. The primary end point was 1-year survival rate. The secondary end points included overall survival, progression-free survival (PFS), response rate (RR), and safety.
Results
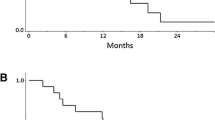
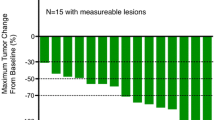
A total 22 patients from seven centers were enrolled. In the 20 patients evaluable for analysis, the 1-year survival rate was 70 % (95 % confidence interval (CI) 49.9–90.1 %), and median survival time, PFS, and RR were 15.3, 7.5 months and 41.2 %, respectively. Major grade 3/4 adverse events were neutropenia (30 %), anorexia (30 %), leukopenia (25 %), fatigue (20 %), and anemia (15 %).
Conclusions
Five-weekly S-1 and cisplatin combined with trastuzumab showed effective with favorable safety profile in patients with HER2-positive AGC.


Similar content being viewed by others
References
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, Bodenstein H, Schmoll HJ, Bleiberg H, Nordlinger B, Couvreur ML, Baron B, Wils JA (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18(14):2648–2657
Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, Joffe JK, Mackean M, Mansi J, Leahy M, Hill A, Oates J, Rao S, Nicolson M, Hickish T (1999) Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 80(1–2):269–272. doi:10.1038/sj.bjc.6690350
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24(31):4991–4997. doi:10.1200/JCO.2006.06.8429
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20(4):666–673. doi:10.1093/annonc/mdn717
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39(1):2–15. doi:10.1093/jjco/hyn127
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu A, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10(11):1063–1069. doi:10.1016/S1470-2045(09)70259-1
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28(9):1547–1553. doi:10.1200/JCO.2009.25.4706
Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A, Ferry D, Moiseyenko V (2013) Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer 49(17):3616–3624. doi:10.1016/j.ejca.2013.07.003
Kataoka H (2009) EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci 56(3):148–153. doi:10.1016/j.jdermsci.2009.10.002
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19(9):1523–1529. doi:10.1093/annonc/mdn169
Shimoyama S (2014) Unraveling trastuzumab and lapatinib inefficiency in gastric cancer: future steps (review). Mol Clin Oncol 2(2):175–181. doi:10.3892/mco.2013.218
Hudis CA (2007) Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med 357(1):39–51. doi:10.1056/NEJMra043186
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, Komatsu Y, Doki Y, Tsujinaka T, Furukawa H (2014) Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 110(5):1163–1168. doi:10.1038/bjc.2014.18
Boo YJ, Park SS, Kim JH, Mok YJ, Kim SJ, Kim CS (2007) Gastric neuroendocrine carcinoma: clinicopathologic review and immunohistochemical study of E-cadherin and Ki-67 as prognostic markers. J Surg Oncol 95(2):110–117. doi:10.1002/jso.20616
Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K (2013) Neuroendocrine carcinoma of the stomach: clinicopathological and immunohistochemical evaluation. Med Mol Morphol 46(1):34–40. doi:10.1007/s00795-012-0006-8
Ryu MH, Baba E, Lee KH, Park YI, Boku N, Hyodo I, Nam BH, Esaki T, Yoo C, Ryoo BY, Song EK, Cho SH, Kang WK, Yang SH, Zang DY, Shin DB, Park SR, Shinozaki K, Takano T, Kang YK, Investigators SOSs (2015) Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol 26(10):2097–2101. doi:10.1093/annonc/mdv316
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol 26(1):141–148. doi:10.1093/annonc/mdu472
Acknowledgments
We wish to thank Professor Suzuki and Dr. Nishiyama for statistical analyses advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataoka, H., Mori, Y., Shimura, T. et al. A phase II prospective study of the trastuzumab combined with 5-weekly S-1 and CDDP therapy for HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol 77, 957–962 (2016). https://doi.org/10.1007/s00280-016-3013-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3013-y