Abstract
Purpose
The fully human monoclonal antibody nivolumab binds to the programmed death-1 (PD-1) receptor, blocking interactions between PD-1 and its ligands on tumor cells and preventing T cell exhaustion in patients with cancer. The potential for corrected QT interval (QTc) prolongation was assessed in a subset of patients enrolled in a phase 2 dose-ranging study of nivolumab.
Methods
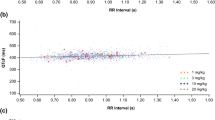
Triplicate 12-lead electrocardiograms (ECGs) obtained predose and post-dose were assessed by an independent ECG core laboratory. QTc derived from Fridericia’s formula (QTcF) was evaluated by central tendency, categorical, and concentration–response analyses.
Results
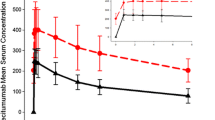
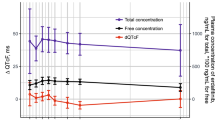
No patients had QTcF intervals or changes from baseline in QTcF (ΔQTcF) exceeding prespecified thresholds indicating borderline or prolonged QTcF (>480 ms) or ΔQTcF (>60 ms). Among 146 patients randomized to nivolumab 0.3, 2.0, or 10.0 mg/kg every 3 weeks, the maximum increases in mean (±SD) ∆QTcF at any time point were 4.9 (±13.4), 1.2 (±10.1), and 2.0 (±8.9) ms, respectively. There was no relationship between ∆QTcF and nivolumab serum concentration and no association between predicted maximum ∆QTcF and mean maximum nivolumab concentration in any dosage group.
Conclusion
Results of these intensive ECG analyses indicate that nivolumab has no clinically meaningful effect on QTc interval when administered at doses up to 10.0 mg/kg.



Similar content being viewed by others
References
Shih K, Arkenau HT, Infante JR (2014) Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 74:1993–2013
Deeks ED (2014) Nivolumab: a review of its use in patients with malignant melanoma. Drugs 74:1233–1239
Weber JS, D’Angelo SP, Minor D et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384
Long GV, Atkinson V, Ascierto PA et al (2015) Nivolumab improved survival vs dacarbazine in patients with untreated advanced melanoma. J Transl Med 13:O6
Topalian SL, Sznol M, McDermott DF et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330
Rizvi NA, Mazieres J, Planchard D et al (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16:257–265
Motzer RJ, Rini BI, McDermott DF et al (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33:1430–1437
McDermott DF, Drake CG, Sznol M et al (2015) Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 33:2013–2020
Ansell SM, Lesokhin AM, Borrello I et al (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311–319
Opdivo® [prescribing information] Bristol-Myers Squibb, Princeton. Revised Nov 2015
Rodriguez I, Erdman A, Padhi D et al (2010) Electrocardiographic assessment for therapeutic proteins—scientific discussion. Am Heart J 160:627–634
Rock EP, Finkle J, Fingert HJ et al (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157:827–836
Giorgi MA, Bolanos R, Gonzalez CD, Di Girolamo G (2010) QT interval prolongation: preclinical and clinical testing arrhythmogenesis in drugs and regulatory implications. Curr Drug Saf 5:54–57
Vargas HM, Bass AS, Breidenbach A et al (2008) Scientific review and recommendations on preclinical cardiovascular safety evaluation of biologics. J Pharmacol Toxicol Methods 58:72–76
Roden DM (2008) Cellular basis of drug-induced torsades de pointes. Br J Pharmacol 154:1502–1507
Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK (2015) QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer 112:296–305
Shah RR, Morganroth J, Shah DR (2013) Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf 36:295–316
Shah RR (2007) Cardiac repolarisation and drug regulation: assessing cardiac safety 10 years after the CPMP guidance. Drug Saf 30:1093–1110
Deeken JF, Shimkus B, Liem A et al (2013) Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol 71:1473–1483
Garg A, Li J, Clark E et al (2013) Exposure-response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol 72:1133–1141
Fridericia LS (1920) The duration of systole in an electrocardiogram in normal humans and patients with heart disease. Acta Med Scand 53:469–486
Bazett HC (1920) An analysis of the time-relations of electrocardiograms. Heart 7:353–370
Agrawal S, Williams D, Waxman I, Lambert A, Roy A, Darbenzio R (2015) Absence of QT prolongation effect by nivolumab or ipilimumab in patients with solid tumors. Clin Pharmacol Ther 97:PII–117
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH) Steering Committee. ICH harmonised tripartite guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, E14. Geneva, Switzerland, ICH, 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 20 Oct 2015
Acknowledgments
Editorial support was provided by Blair Jarvis of inScience Communications, Springer Healthcare (Philadelphia, PA, USA), and funded by Bristol-Myers Squibb (Princeton, NJ, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of and own stock in Bristol-Myers Squibb.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agrawal, S., Waxman, I., Lambert, A. et al. Evaluation of the potential for QTc prolongation in patients with solid tumors receiving nivolumab. Cancer Chemother Pharmacol 77, 635–641 (2016). https://doi.org/10.1007/s00280-016-2980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2980-3