Abstract
Background
The oral PARP inhibitor olaparib has shown efficacy in patients with BRCA-mutated cancer. This Phase I, open-label, three-part study (Parts A–C) in patients with advanced solid tumours evaluated the effect of food on the pharmacokinetics (PK) of olaparib when administered in tablet formulation.
Methods
PK data were obtained in Part A using a two-treatment period crossover design; single-dose olaparib 300 mg (two 150 mg tablets) was administered in two prandial states: fasted and fed. In Part B, patients received olaparib tablets (300 mg bid) for 5 days under fasting conditions; in Part C, patients were allowed continued access to olaparib. Safety was assessed throughout, with data reported for Parts A and B.
Results
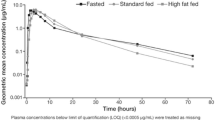
A total of 60 and 56 patients were evaluable for safety and PK analyses, respectively; 57 patients entered Part B. Rate of olaparib absorption was slower in the presence of food (t max delayed by 2.5 h), resulting in a statistically significant ~21 % decrease in peak plasma exposure (C max) [ratio of geometric means (90 % CI), 0.79 (0.72, 0.86)] but only a marginal increase in olaparib absorption (AUC0–∞) [ratio of geometric means (90 % CI), 1.08 (1.01, 1.16)]. The point estimate and 90 % CI for the AUC0–∞ treatment ratio were within pre-defined bioequivalence limits (0.80–1.25). Adverse event data were consistent with the known safety profile of olaparib.
Conclusions
Results of this study showed that a high-fat meal decreases the rate of absorption and peak exposure to olaparib 300 mg tablets, although in the absence of an effect on the extent of olaparib absorption.

Similar content being viewed by others
References
Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo HB, Smith GC, Martin NM, Lau A, O’Connor MJ, Jonkers J (2008) Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res 14:3916–3925
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Connor MJ, Martin NM, Borst P, Jonkers J (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 105:17079–17084
Marchetti C, Imperiale L, Gasparri ML, Palaia I, Pignata S, Boni T, Bellati F, Benedetti PP (2012) Olaparib, PARP1 inhibitor in ovarian cancer. Expert Opin Investig Drugs 21:1575–1584
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376:235–244
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, MacPherson E, Carmichael J, Oza A (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12:852–861
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Steiner M, Loman N, Bowen K, Fielding A, Domchek SM (2014) Olaparib monotherapy in patients with advanced cancer and a germ-line BRCA1/2 mutation. J Clin Oncol. doi:10.1200/JCO.2014.56.2728 (Nov 3 [Epub ahead of print])
Oza A, Cibula D, Benzaquen AO, Poole C, Mathijssen RHJ, Sonke GS, Colombo N, Spacek J, Vuylsteke P, Hirte H, Mahner S, Plante M, Schmalfeldt B, Mackay H, Rowbottom J, Lowe E, Dougherty B, Barrett JC, Friedlander M (2015) Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 16:87–97
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira Frommer R, Safra T, Matei D, MacPherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382–1392
Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett J, Matulonis U (2014) Randomized trial of olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis by BRCA mutation status. Lancet Oncol 15:852–861
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JHM, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134
Molife R, Kaye S, Forster M, Ransom M, Greystoke A, Middleton M, Pwint T, McCormack P, Swailand H, Carmichael J (2010) A phase I study to determine the comparative bioavailability of two different oral formulations of the PARP inhibitor, olaparib (AZD2281), in patients with advanced solid tumors. J Clin Oncol 28(7S):abst 2599
Mateo J, Friedlander M, Sessa C, Leunen K, Nicum S, Gourley C, Fielding A, Bowen K, Kaye S, Molife LR (2013) Administration of continuous/intermittent olaparib in ovarian cancer patients with a germline BRCA1/2 mutation to determine an optimal dosing schedule for the tablet formulation. Eur J Cancer 49(2 Suppl):abst 801
Molife LR, Mateo J, McGoldrick T, Krebs M, Drew Y, Banerjee SN, Nicum S, Ranson M, Rustin GJ, Sessa C, Plummer R, Leunen K, Friedlander M, Swaisland H, Burke W, McCormack P, Pemberton K, Tchakov I, Kaye SB, Gourley C (2012) Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations. J Clin Oncol 30(15S):abst 3048
AstraZeneca. Global policy: bioethics. 2015. http://www.astrazeneca.com/Responsibility/Code-policies-standards/Our-global-policies
U.S. Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2002) Guidance for industry: food-effect bioavailability and fed bioequivalence studies. FDA
Rolfo C, Swaisland H, Leunen K, Rutten A, Soetekouw P, Slater S, Verheul HM, Fielding A, So K, Bannister W, Dean E (2015) Effect of food on the pharmacokinetics of olaparib after oral dosing of the capsule formulation in patients with advanced solid tumors. Adv Ther 32:510–522
Acknowledgments
The authors would like to thank all patients who consented to participate in this study. We also acknowledge Gil Morrison (Covance) for analysis of the PK data, and Quintiles for conducting the study and associated data management activities. This study was sponsored by AstraZeneca. Medical writing assistance was provided by Claire Routley Ph.D. from Mudskipper Business Ltd, funded by AstraZeneca. The UK Centres participating in this research receive funding from Cancer Research UK (CRUK) and the Department of Health as Experimental Cancer Medicine Centres.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RP has received institutional remuneration from AstraZeneca and has received funding from AstraZeneca for chairing an advisory board. GJ has received remuneration from Novartis, Celgene, and Roche, funding from Novartis, MSD, and Roche, and has consulted/had an advisory role for Novartis and Celgene. LRM has received funding from AstraZeneca. JDG has received funding from AstraZeneca for attendance at a national advisory board. AF is an employee of, and owns stock in, AstraZeneca. HS was formally an employee of AstraZeneca and owns stock in AstraZeneca. KS is a contractor for AstraZeneca. KL, CvH, DN, ML, JS, MMS, PS, and WB have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
HS was an employee of AstraZeneca while the study was conducted.
Rights and permissions
About this article
Cite this article
Plummer, R., Swaisland, H., Leunen, K. et al. Olaparib tablet formulation: effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother Pharmacol 76, 723–729 (2015). https://doi.org/10.1007/s00280-015-2836-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2836-2