Abstract
Background
Single-agent gemcitabine (GEM) has been considered for many years as the standard first-line treatment for advanced pancreatic cancer. However, recently, several studies reported encouraging activity and good tolerability for some combination regimens. Considering the apparently non-overlapping toxicity and the proved individual efficacy of GEM, oxaliplatin (l-OHP), and capecitabine (CAP), this randomized phase II study compared the activity and safety of the combination GEM, l-OHP, and CAP (GEMOXEL) versus GEM alone, in patients with metastatic pancreatic cancer.
Materials and methods
The treatment in GEMOXEL arm consisted of GEM 1,000 mg/m2 as a 30-min intravenous infusion on days 1, 8, 15, 22, l-OHP 100 mg/m2 i.v. on day 2, and CAP 1,500 mg/m2/day in two divided doses on days 1–14, every 21 days (one cycle). In both treatment groups, GEM was administered weekly for seven consecutive weeks followed by 1-week rest for the first 8 weeks, and thereafter, GEM was continued on days 1, 8, 15, every 28 days. Chemotherapy was administered until disease progression or unacceptable toxicity.
Results
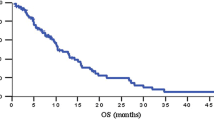
Sixty-seven patients were enrolled in the study. Thirty-four were randomly assigned to GEMOXEL and 33 to GEM. At 4 months, disease control rate was 79.4 % with GEMOXEL versus 45.4 % with GEM (p = 0.004). The median progression-free survival was 6.8 months (95 % CI 5.3–7.3 months) in GEMOXEL arm and 3.7 months (95 % CI 2.9–4.7 months) in GEM arm (p < 0.001). The median OS was 11.9 months (95 % CI 10.6–12.9 months) in GEMOXEL arm and 7.1 months (95 % CI 5.5–9.1 months) in GEM arm (p < 0.001). Hematologic and non-hematologic toxicity was more severe with combination chemotherapy, yet still tolerable. No grade 4 adverse events were observed with either regimen.
Conclusion
GEMOXEL regimen seemed to be safe and more efficient than the standard therapy with GEM alone in the treatment of metastatic pancreatic cancer.


Similar content being viewed by others
References
Kris MG, Benowitz SI, Adams S, Diller L, Ganz P, Kahlenberg MS, Le QT, Markman M, Masters GA, Newman L, Obel JC, Seidman AD, Smith SM, Vogelzang N, Petrelli NJ, Clinical Cancer Advances (2010) Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 28(36):5327–5347
Stathis A, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7(3):163–172
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362(17):1605–1617
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, Faroux R, Lepere C, de Gramont A (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23(15):3509–3516
Heinemann V, Labianca R, Hinke A, Louvet C (2007) Increased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicenter study. Ann Oncol 10:1652–1659
Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, Ciaparrone M, Cavanna L, Giuliani F, Maiello E, Testa A, Pederzoli P, Falconi M, Gallo C, Di Maio M, Perrone F (2010) Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28(10):1645–1651
Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T, Iveson T, O’Brien M, Tebbutt N, Harrington A, Hill M (2002) Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol 20(14):3130–3136
Chau I, Cunningham D, Russell C, Norman AR, Kurzawinski T, Harper P, Harrison P, Middleton G, Daniels F, Hickish T, Prendeville J, Ross PJ, Theis B, Hull R, Walker M, Shankley N, Kalindjian B, Murray G, Gillbanks A, Black J (2006) Gastrazole (JB95008), a novel CCK2/gastrin receptor antagonist, in the treatment of advanced pancreatic cancer: results from two randomised controlled trials. Br J Cancer 94(8):1107–1115
Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Köhne CH, Mingrone W, Stemmer SM, Tàmas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W, Swiss Group for Clinical Cancer Research, Central European Cooperative Oncology Group (2007) Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 25(16):2212–2217
Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, Falk S, Crellin A, Adab F, Thompson J, Leonard P, Ostrowski J, Eatock M, Scheithauer W, Herrmann R, Neoptolemos JP (2009) Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 27(33):5513–5518
Tan BR, Brenner WS, Picus J, Marsh S, Gao F, Fournier C, Fracasso PM, James J, Yen-Revollo JL, McLeod HL (2008) Phase I study of biweekly oxaliplatin, gemcitabine and capecitabine in patients with advanced upper gastrointestinal malignancies. Ann Oncol 19(10):1742–1748
Hess V, Pratsch S, Potthast S, Lee L, Winterhalder R, Widmer L, Cescato C, Lohri A, Jost L, Stillhart P, Pestalozzi B, Herrmann R (2010) Combining gemcitabine, oxaliplatin and capecitabine (GEMOXEL) for patients with advanced pancreatic carcinoma (APC): a phase I/II trial. Ann Oncol 21(12):2390–2395
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of Unicancer, PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain 1:277–299
De Benedittis G, Massei R, Nobili R, Pieri A (1988) The Italian Pain Questionnaire. Pain 33:53–62
McCormack A, Hunter-Smith D, Piotrowski ZH, Grant M, Kubik S, Kessel K (1992) Analgesic use in home hospice cancer patients. J Fam Pract 34:160–164
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Philip PA, Mooney M, Jaffe D, Eckardt G, Moore M, Meropol N, Emens L, O’Reilly E (2009) Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 27:5660–5669
Li Q, Yan H, Liu W, Zhen H, Yang Y, Cao B (2014) Efficacy and safety of gemcitabine–fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomized controlled trials. PLoS One 9(8):e104346
Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke A, Schmiegel W, Schmoll HJ, Porschen R (2008) Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol 26(36):5910–5917
Acknowledgments
The authors wish to thank all the members of the local Oncology Group on Gastrointestinal Tumors and the following oncologists and surgeons who substantially contributed to recruit eligible patients and made this study possible: Dr. Franco Papi, Dr Vinno Savelli, General Surgery, University of Siena; Dr. Carmine Andrea Calvanese, Medical Oncology, Salerno; Dr. G. Giusti, USL 10, Pisa, Italy; Dr. G. Vegni, Volterra Hospital; Dr.ssa T. Cerri Vestri, USL Arezzo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrioli, R., Roviello, G., Fiaschi, A.I. et al. Gemcitabine, oxaliplatin, and capecitabine (GEMOXEL) compared with gemcitabine alone in metastatic pancreatic cancer: a randomized phase II study. Cancer Chemother Pharmacol 75, 683–690 (2015). https://doi.org/10.1007/s00280-015-2683-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2683-1