Abstract
Purpose
The purpose of this multicenter phase II study was to evaluate the efficacy and safety of a combination of irinotecan, 5-fluorouracil (5-FU), and leucovorin (FOLFIRI) plus bevacizumab as first-line chemotherapy in Japanese patients with metastatic colorectal cancer.
Methods
Patients with metastatic colorectal cancer were eligible for enrollment. On day 1 of a 14-day cycle, patients received bevacizumab 5 mg/kg, irinotecan 150 mg/m2, and l-leucovorin 200 mg/m2 as an intravenous infusion, followed by 5-FU 400 mg/m2 as an intravenous bolus and then 5-FU 2,400 mg/m2 as an 46-h intravenous infusion. This treatment cycle was repeated. The primary endpoint was progression-free survival (PFS).
Results
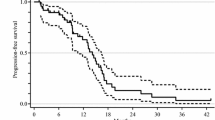
We enrolled 40 patients, but one withdrew consent before starting treatment. The remaining 39 patients received a total of 509 cycles of FOLFIRI plus bevacizumab (median 11 per patient; range 1–30). The median PFS was 11.5 months, the median overall survival (OS) was 22.0 months, and the 1-year OS rate was 81.8 %. All 39 patients had adverse events. Grade 3 or 4 neutropenia and stomatitis occurred in 21 (53.9 %) and 4 (10.3 %) patients, respectively.
Conclusion
Our results suggest that FOLFIRI plus bevacizumab is a clinically effective regimen with a manageable toxicity profile as first-line chemotherapy in patients with metastatic colorectal cancer.



Similar content being viewed by others
References
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25:4779–4786. doi:10.1200/JCO.2007.11.3357
Fuchs CS, Marshall J, Barrueco J (2008) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol 26:689–690. doi:10.1200/JCO.2007.15.5390
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl cancer 92:205–216
Kochi M, Ichikawa W, Meguro E, Shibata H, Fukui T, Nagase M, Hoshino Y, Takeuchi M, Fujii M, Nakajima T (2011) Phase II study of FOLFOX4 with “wait and go” strategy as first-line treatment for metastatic colorectal cancer. Cancer Chemother Pharmacol 68:1215–1222. doi:10.1007/s00280-011-1605-0
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30. doi:10.1200/JCO.2004.09.046
Andre T, Boni C, Mounedji-Boudiaf Ll, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Philip C, Bridgewater J, Tabah-Fisch I, de Gramont A (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351. doi:10.1056/NEJMoa032709
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214. doi:10.1200/JCO.2004.11.037
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL, Irinotecan Study Group (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 343:905–914. doi:10.1056/NEJM200009283431302
Ferrara N (1999) Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77(7):527–543
Gordon MS, Margolin K, Talpaz M, Sledge GW Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D (2001) Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19(3):843–850
Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J (2001) Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol 19(3):851–856
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019. doi:10.1200/JCO.2007.14.9930
Acknowledgments
We are grateful to W. Koizumi, Y. Shimada, and S. Maetani for their kind advice and to T. Sasaki, H. Takahashi, and A. Kawano, who served on the independent review committee. We also thank S. Koyama and Y. Kakehashi for their data management. This study has been presented in part at the 9th Annual Meeting of the Japanese Society of Medical Oncology, Yokohama, Japan, 2011. This study was supported by the Japan Clinical Cancer Research Organization (JACCRO). The authors are indebted to Prof. J. Patrick Barron of the Department of International Medical Communications of Tokyo Medical University for his review of this manuscript.
Conflict of interest
The authors declared that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The following investigators participated in the study: Mitsugu Kochi, Ken Hagiwara (Nihon University School of Medicine, Tokyo, Japan); Akinori Takagane, (Hakodate Goryokaku Hospital, Hokkaido, Japan); Tatsuya Aoki (Tokyo Medical University Hospital, Tokyo, Japan); Takao Takahashi (Department of Surgical Oncology, Gifu University Graduate School of Medicine); Katsuji Hironaka (Department of Clinical Oncology, Kawasaki Medical School); Futoshi Teranishi (Midori Municipal Hospital, Aichi, Japan); and Fumihiko Osuka (Fukushima Medical University, Fukushima, Japan).
Rights and permissions
About this article
Cite this article
Kochi, M., Akiyama, Y., Aoki, T. et al. FOLFIRI plus bevacizumab as a first-line treatment for Japanese patients with metastatic colorectal cancer: a JACCRO CC-03 multicenter phase II study. Cancer Chemother Pharmacol 72, 1097–1102 (2013). https://doi.org/10.1007/s00280-013-2292-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2292-9