Abstract
Background
A growing number of preclinical studies have demonstrated that curcumin could be a promising anticancer drug; however, poor bioavailability has been the major obstacle for its clinical application. To overcome this problem, we developed a new form of curcumin (Theracurmin®) and reported high plasma curcumin levels could be safely achieved after a single administration of Theracurmin® in healthy volunteers. In this study, we aimed to evaluate the safety of repetitive administration of Theracurmin® in cancer patients.
Methods
Pancreatic or biliary tract cancer patients who failed standard chemotherapy were eligible for this study. Based on our previous pharmacokinetic study, we selected Theracurmin® containing 200 mg of curcumin (Level 1) as a starting dose, and the dose was safely escalated to Level 2, which contained 400 mg of curcumin. Theracurmin® was orally administered every day with standard gemcitabine-based chemotherapy. In addition to safety and pharmacokinetics data, NF-κB activity, cytokine levels, efficacy, and quality-of-life score were evaluated.
Results
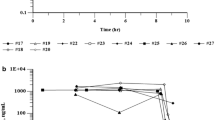
Ten patients were assigned to level 1 and six were to level 2. Peak plasma curcumin levels (median) after Theracurmin® administration were 324 ng/mL (range, 47–1,029 ng/mL) at Level 1 and 440 ng/mL (range, 179–1,380 ng/mL) at Level 2. No unexpected adverse events were observed and 3 patients safely continued Theracurmin® administration for >9 months.
Conclusions
Repetitive systemic exposure to high concentrations of curcumin achieved by Theracurmin® did not increase the incidence of adverse events in cancer patients receiving gemcitabine-based chemotherapy.

Similar content being viewed by others
References
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH (2010) Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 70:3606–3617
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC (2011) Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 4:1158–1171
Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A (2007) Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 5:3
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–2900
Cho JW, Lee KS, Kim CW (2007) Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med 19:469–474
Corson TW, Crews CM (2007) Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell 130:769–774
Das RK, Kasoju N, Bora U (2010) Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 6:153–160
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R (2008) Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14:4491–4499
Du B, Jiang L, Xia Q, Zhong L (2006) Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 52:23–28
Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G (2010) Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer 62:1137–1141
Fabre JM, Burgel JS, Navarro F, Boccarat G, Lemoine C, Domergue J (1999) Delayed gastric emptying after pancreaticoduodenectomy and pancreaticogastrostomy. Eur J Surg 165:560–565
Fayers P, Aaronson N, Bjordal K, Groenvoold M, Curran D (2001) Bottomley A (2001) EORTC QLQ-C30 Scoring Manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels
Fox E, Curt GA, Balis FM (2002) Clinical trial design for target-based therapy. Oncologist 7:401–409
Gescher AJ (2012) Dose escalation and pharmacokinetic study of nanoparticle curcumin… by Kanai et al., CCP 69:65–70, 2012. Cancer Chemother Pharmacol 70:487
Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K (2009) Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology 214:33–39
Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A (2001) Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res 61:1058–1064
Kanai M, Imaizumi A, Otsuka Y, Sasaki H, Hashiguchi M, Tsujiko K, Matsumoto S, Ishiguro H, Chiba T (2012) Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol 69:65–70
Kanai M, Matsumoto S, Otsuka Y, Fukuda M, Imaizumi A (2012) In response. Cancer Chemother Pharmacol 70:489
Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y, Yanagihara K, Yazumi S, Chiba T, Guha S, Aggarwal BB (2011) A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 68:157–164
Kanai M, Yoshimura K, Tsumura T, Asada M, Suzuki C, Niimi M, Matsumoto S, Nishimura T, Nitta T, Yasuchika K, Taura K, Mori Y, Hamada A, Inoue N, Tada S, Yanagihara K, Yazumi S, Osaki Y, Chiba T, Ikai I, Fukushima M, Uemoto S, Hatano E (2010) A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 67:1429–1434
Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB (2007) Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 67:3853–3861
Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R (2004) Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 101:2351–2362
Li L, Braiteh FS, Kurzrock R (2005) Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 104:1322–1331
LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang MB (2005) Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 11:6994–7002
Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ (2007) Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 60:171–177
Meyers CA, Albitar M, Estey E (2005) Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 104:788–793
Morimoto T, Sunagawa Y, Fujita M, Hasegawa K (2010) Novel heart failure therapy targeting transcriptional pathway in cardiomyocytes by a natural compound, curcumin. Circ J 74:1059–1066
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dorken B, Riess H, Oettle H (2011) Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 47:1676–1681
Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M (2007) Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem 55:538–544
Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T (2011) Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 34(5):660-665
Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, Iwabuchi Y, Shibata H (2011) Curcumin analog GO-Y030 is a novel inhibitor of IKKbeta that suppresses NF-kappaB signaling and induces apoptosis. Cancer Sci 102:1045–1051
Seruga B, Zhang H, Bernstein LJ, Tannock IF (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8:887–899
Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN (2009) Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci 37:223–230
Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10:6847–6854
Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer 41:1955–1968
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–545
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733–742
Uddin S, Hussain AR, Manogaran PS, Al-Hussein K, Platanias LC, Gutierrez MI, Bhatia KG (2005) Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene 24:7022–7030
Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE (2008) Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 17:1411–1417
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ (2005) The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol 518:40–46
Acknowledgments
We thank Kazuyuki Miura and Megumi Horikawa for their contributions to data management and Yasuko Nakagawa for her contribution to sample collection and preparation. This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (24590655) and the Japanese Research Foundation for Clinical Pharmacology.
Conflicts of interest
A. Imaizumi is a consultant to Theravalues Corporation, and Y. Otsuka is an employee of Theravalues Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanai, M., Otsuka, Y., Otsuka, K. et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin®) in cancer patients. Cancer Chemother Pharmacol 71, 1521–1530 (2013). https://doi.org/10.1007/s00280-013-2151-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2151-8