Abstract
Purpose
Sorafenib and everolimus are both active against neuroendocrine tumors (NET). Because of potential synergy between VEGF pathway and mTOR inhibitors, we performed a phase I study to evaluate the safety and feasibility of combining sorafenib and everolimus in patients with advanced NET.
Methods
Patients were treated with everolimus 10 mg daily in combination with sorafenib (dose level 1: 200 mg twice daily; dose level 2: 200 mg per morning, 400 mg per evening) using standard phase I dose escalation design. Dose-limiting toxicity (DLT) was defined within the first cycle (28 days) of therapy. Treatment was continued until tumor progression, unacceptable toxicity, or withdrawal of consent. Twelve additional patients were treated at the maximum tolerated dose (MTD) level to further characterize safety and a preliminary assessment of activity.
Results
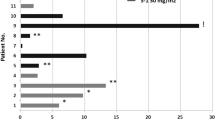
One patient in Cohort 1 experienced DLT (grade 3 skin rash); the cohort was expanded to 6 patients with no further DLTs. All 3 patients in Cohort 2 experienced DLT, consisting of thrombocytopenia, hand–foot skin reaction, and rash/allergic reaction. Sorafenib 200 mg twice daily in combination with everolimus 10 mg daily was established as the MTD. Independently reviewed best objective responses revealed that 62 % of patients had some degree of tumor shrinkage. By RECIST, we observed partial response in 1 patient, stable disease in 13 patients, and progressive disease in 3 patients.
Conclusion
Sorafenib 200 mg twice daily with everolimus 10 mg daily represents the MTD of this combination in patients with advanced NET. While the combination is active, toxicity concerns may preclude more widespread use.

Similar content being viewed by others
References
Vignot S, Faivre S, Aguirre D et al (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16:525–537
Yao JC, Shah MH, Ito T et al (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514–523
Pavel ME, Hainsworth JD, Baudin E et al (2011) Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 378:2005–2012
Bowen KA, Silva SR, Johnson JN et al (2009) An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J Gastrointest Surg 13:1773–1780
Silva SR, Bowen KA, Rychahou PG et al (2011) VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int J Cancer 128:1045–1056
Fjallskog ML, Hessman O, Eriksson B et al (2007) Upregulated expression of PDGF receptor beta in endocrine pancreatic tumors and metastases compared to normal endocrine pancreas. Acta Oncol 46:741–746
Fjallskog ML, Lejonklou MH, Oberg KE et al (2003) Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res 9:1469–1473
Hansel DE, Rahman A, Hermans J et al (2003) Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Mod Pathol 16:652–659
Raymond E, Dahan L, Raoul JL et al (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501–513
Hobday TJ, Rubin J, Holen K et al. (2007) MC044 h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): a Phase II Consortium (P2C) study. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I 25:Abstract 4504
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Mir O, Coriat R, Boudou-Rouquette P et al (2012) Sorafenib-induced diarrhea and hypophosphatemia: mechanisms and therapeutic implications. Ann Oncol 23:280–281
Molina AM, Feldman DR, Voss MH et al (2012) Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer 118:1868–1876
Harzstark AL, Small EJ, Weinberg VK et al (2011) A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer 117:4194–4200
Hobday T, Qin R, Reidy D et al. (2012) Multicenter phase II trial of temsirolimus (TEM) and bevacizumab (BEV) in pancreatic neuroendocrine tumor (PNET). J Clin Oncol, 2012 ASCO Annual Meeting Proceedings 30:(suppl 4; abstr 260)
Yao J, Phan A, Fogleman D et al (2010) Randomized run-in study of bevacizumab (B) and everolimus (E) in low- to intermediate-grade neuroendocrine tumors (LGNETs) using perfusion CT as functional biomarker. J Clin Oncol, 2010 ASCO Annual Meeting Proceedings 28:15s:(suppl; abstr 4002)
Acknowledgments
The authors gratefully acknowledge support from the Saul and Gitta Kurlat fund for neuroendocrine tumor research. This work was supported by Novartis, Bayer, and Onyx Pharmaceuticals.
Conflict of interest
J. Chan: research funding from Novartis, Schering-Plough/Merck, Bayer, and Onyx Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, J.A., Mayer, R.J., Jackson, N. et al. Phase I study of sorafenib in combination with everolimus (RAD001) in patients with advanced neuroendocrine tumors. Cancer Chemother Pharmacol 71, 1241–1246 (2013). https://doi.org/10.1007/s00280-013-2118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2118-9