Abstract
Background
A phase I dose-escalation study was performed to investigate the safety and pharmacokinetics of the combination of S-1 and gefitinib in patients with pulmonary adenocarcinoma who had failed previous chemotherapy.
Methods
Patients received gefitinib at a fixed daily oral dose of 250 mg, and S-1 was administered on days 1–14 every 21 days at doses starting at 60 mg/m2 (level 1) and escalating to 80 mg/m2 (level 2). The primary end point of the study was determination of the recommended dose for S-1 given in combination with a fixed dose of gefitinib.
Results
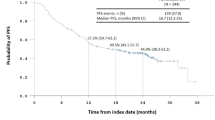
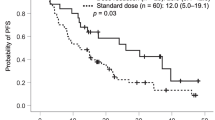
Twenty patients were enrolled in the study. Two of the first six patients at dose level 2 experienced a dose-limiting toxicity (elevation of alkaline phosphatase of grade 3 in one patient; elevations of aspartate and alanine aminotransferases of grade 3 in the other). The recommended dose was thus determined as level 2, and an additional 11 patients were assigned to this level. All observed adverse events were well managed. The response rate was 50 % (10 of 20 patients), and the median progression-free survival (PFS) and overall survival times were 10.5 and 21.2 months, respectively. In EGFR mutation–positive patients (n = 9), seven patients achieved an objective response and the median PFS was 12.4 months, whereas none with wild-type EGFR (n = 6) responded. No pharmacokinetic interaction between S-1 and gefitinib was detected.
Conclusions
The combination of S-1 and gefitinib is well tolerated and appears to possess activity against EGFR mutation–positive NSCLC.
Similar content being viewed by others
References
Kosaka T, Yatabe Y, Endoh H et al (2004) Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 64:8919–8923
Shigematsu H, Lin L, Takahashi T et al (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339–346
Okamoto I, Fukuoka M (2009) S-1: a new oral fluoropyrimidine in the treatment of patients with advanced non-small-cell lung cancer. Clin Lung Cancer 10:290–294
Hirata K, Horikoshi N, Aiba K et al (1999) Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5:2000–2005
Kawahara M, Furuse K, Segawa Y et al (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85:939–943
Okamoto I, Yoshioka H, Morita S et al (2010) Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naive patients with advanced non-small-cell lung cancer: results of a west Japan oncology group study. J Clin Oncol 28:5240–5246
Okabe T, Okamoto I, Tsukioka S et al (2008) Synergistic antitumor effect of S-1 and the epidermal growth factor receptor inhibitor gefitinib in non-small cell lung cancer cell lines: role of gefitinib-induced down-regulation of thymidylate synthase. Mol Cancer Ther 7:599–606
Okabe T, Okamoto I, Tsukioka S et al (2009) Addition of S-1 to the epidermal growth factor receptor inhibitor gefitinib overcomes gefitinib resistance in non-small cell lung cancer cell lines with MET amplification. Clin Cancer Res 15:907–913
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Matsushima E, Yoshida K, Kitamura R (1997) Determination of S-1 (combined drug of tegafur, 5-chloro-2,4-dihydroxypyridine and potassium oxonate) and 5-fluorouracil in human plasma and urine using high-performance liquid chromatography and gas chromatography-negative ion chemical ionization mass spectrometry. J Chromatogr B Biomed Sci Appl 691:95–104
Maruyama R, Nishiwaki Y, Tamura T et al (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26:4244–4252
Ikeda K, Yoshisue K, Matsushima E et al (2000) Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res 6:4409–4415
Park JY, Kim KA (2003) Inhibitory effect of 5-fluorouracil on human cytochrome P(450) isoforms in human liver microsomes. Eur J Clin Pharmacol 59:407–409
McKillop D, McCormick AD, Miles GS et al (2004) In vitro metabolism of gefitinib in human liver microsomes. Xenobiotica 34:983–1000
McKillop D, McCormick AD, Millar A et al (2005) Cytochrome P450-dependent metabolism of gefitinib. Xenobiotica 35:39–50
Nakagawa K, Tamura T, Negoro S et al (2003) Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 14:922–930
Ranson M, Hammond LA, Ferry D et al (2002) ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol 20:2240–2250
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiyota, H., Okamoto, I., Takeda, M. et al. Phase I and pharmacokinetic study of gefitinib and S-1 combination therapy for advanced adenocarcinoma of the lung. Cancer Chemother Pharmacol 71, 859–865 (2013). https://doi.org/10.1007/s00280-013-2077-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2077-1