Abstract
Purpose
Liposome and immunoliposome formulations of two vinca alkaloids, vincristine and vinblastine, were prepared using intraliposomal triethylammonium sucroseoctasulfate and examined for their ability to stabilize the drug for targeted drug delivery in vivo.
Methods
The pharmacokinetics of both the encapsulated drug (vincristine or vinblastine) and liposomal carrier were examined in Sprague Dawley rats, and the in vivo drug release rates determined. Anti-HER2 immunoliposomal vincristine was prepared from a human anti-HER2/neu scFv and studied for targeted cytotoxic activity in cell culture, and antitumor efficacy in vivo.
Results
Nanoliposome formulations of vincristine and vinblastine demonstrated similar pharmacokinetic profiles for the liposomal carrier, but increased clearance for liposome encapsulated vinblastine (t 1/2 = 9.7 h) relative to vincristine (t 1/2 = 18.5 h). Immunoliposome formulations of vincristine targeted to HER2 using an anti-HER2 scFv antibody fragment displayed a marked enhancement in cytotoxicity when compared to non-targeted liposomal vincristine control; 63- or 253-fold for BT474 and SKBR3 breast cancer cells, respectively. Target-specific activity was also demonstrated in HER2-overexpressing human tumor xenografts, where the HER2-targeted formulation was significantly more efficacious than either free vincristine or non-targeted liposomal vincristine.
Conclusions
These results demonstrate that active targeting of solid tumors with liposomal formulations of vincristine is possible when the resulting immunoliposomes are sufficiently stabilized.
Similar content being viewed by others
Introduction
Liposome formulations of vincristine have been in clinical development for over a decade [5, 14, 46, 48]. Unlike anthracyclines, vinca alkaloids are notoriously more difficult to formulate stably in liposomes [10, 44, 48, 51]. Simple pH- or ammonium sulfate gradients are sufficient to load and stabilize doxorubicin in liposomes [1, 7, 15, 30], helping to give rise to long circulating and highly active formulations that include pegylated liposomal doxorubicin (Doxil®, Alza/Johnson&Johnson; Palo Alto, CA). Schedule-dependent drugs such as vincristine [17, 18] would be expected to benefit to an even greater extent from a stable liposome formulation, with controlled release of the drug from the liposomal carrier, thus resulting in an extended duration of exposure of the target cancer tissue to the active drug [2, 12].
In addition, although liposomal vincristine has been most extensively studied in leukemias and lymphomas [31, 32, 43, 44, 46], vincristine, as well as vinblastine, have also shown activity in the treatment of certain solid tumors, including neuroblastomas [38, 39] and melanoma [24, 25]. Accumulation of nanoparticle drug carriers, such as liposomes, in solid tumors is generally governed by a relatively selective, but slow extravasation from a “leaky” tumor microvasculature, as a part of what is commonly referred to as the enhanced permeability and retention (EPR) effect [9, 28]. To fully take advantage of the EPR phenomenon in treating solid tumors, the liposomal formulation must be engineered to retain its active contents for the time needed to effectuate extravasation, or approximately 24–48 h [9, 13, 21], and thus allow for release of the active drug primarily in the near vicinity of the tumor cells.
Finally, newer generations of liposomal delivery technologies include active targeting moieties such as antibodies to direct the liposomal drugs specifically to receptor-overexpressing tumor cells [36, 42]. Because the targeting ligand is not directly conjugated to the drug itself, but instead indirectly to the carrier, stable encapsulation is an absolute requirement to ensure that the drug arrives intact at the target site and reduces exposure to non-target tissues that will arise if the drug becomes bioavailable prematurely while in the circulation [10, 36, 42].
A variety of strategies has been employed to improve the stability of liposomal vincristine formulations. The modification of the lipid composition, and substitution of sphingomyelin for phosphatidylcholine in the formulation, substantially improved the stability of encapsulation for cholesterol-containing formulations [48]. The introduction of fully saturated dihydrosphingomyelin into the formulation has further improved its stability [19]. The use of high drug-to-lipid ratio formulations to increase intraliposomal concentrations of vincristine, and thus reduce its solubility, has also been shown to improve stability [20]. Finally, the use of dextran sulfate to complex vincristine has been used to limit its diffusion from a liposomal carrier, albeit at the expense of decreased antitumor activity [52].
Here, we describe the preparation of novel liposomal vincristine and vinblastine formulations stabilized intraliposomally with the sulfated non-polymeric polyol sucrose octasulfate. These preparations were highly stable in vivo despite the absence of sphingomyelin in the formulation, and even at relatively low vincristine to phospholipid ratios. Immunotargeted versions were prepared through conjugation of a human anti-HER2 scFv to the surface of the carrier, and shown to result in target-specific cytotoxicity in breast cancer cells in culture and improved antitumor efficacy in human breast tumor xenografts in vivo. This proof-of-concept study suggests that immunotargeting of liposomal vincristine to solid tumors is feasible when the nanocarriers are sufficiently stabilized to limit drug leakage in the circulation.
Materials and methods
Materials
1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (PEG-DSPE), and 1,2-distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Maleimide(Polyethylene Glycol)2000] (Ammonium Salt) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol was obtained from Calbiochem (La Jolla, CA). Sucrose octasulfate (sodium salt) was purchased from Toronto Research Chemicals, Inc. (North York, ON, Canada). Sepharose CL-4B and Sephadex G-75 size exclusion resins, Dowex 50W-8X-200 cation exchange resin, triethylamine, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) were all obtained from Sigma-Aldrich (St Louis, MO). [3H]-Cholesteryl hexadecyl ether was purchased from Perkin Elmer (Boston, MA). Vincristine sulfate was purchased from Handetech Development Co. (Houston, TX), and vinblastine sulfate solution USP (American Pharmaceutical Partners, Los Angeles, CA) was purchased from the pharmacy.)
Methods
Liposomal drug preparation
The lipid membranes of vincristine (VCR) and vinblastine (VBL)-loaded liposomes were composed of DSPC, cholesterol, and PEG-DSPE at a molar ratio of 3:2:0.015. The lipids were dissolved in chloroform:methanol (9:1, vol:vol), and dried by rotary evaporation and subsequently for 12 h under vacuum (<100 μTorr). Triethylammmonium sucrose octasulfate (TEA8SOS, sulfate group concentration 0.65 M) was prepared from the sodium salt of sucrose octasulfate using ion exchange chromatography as described previously [11]. The lipids were redissolved in ethanol at 60°C, and mixed with nine volumes of the aqueous TEA8SOS solution while maintaining the same temperature. The resulting mixture was extruded 10 times through polycarbonate membranes (Whatman, Clifton, NJ) having pore sizes of 0.05, 0.8, or 0.1 μm. Unencapsulated TEA8SOS was removed on a low pressure hand poured Sepharose CL-4B size exclusion column (400 mm × 25 mm i.d.), eluted with HEPES-buffered dextrose (5 mM HEPES, 5% dextrose, pH 6.5). Vincristine or vinblastine was added at the indicated ratio of drug to lipid (100–550 g drug salt/mol phospholipid), loading was initiated by adjusting the pH to 6.5 (unless otherwise indicated), and incubating for 30 min at 60°C, after which, the liposome suspension was transferred to an ice bath. The liposomal preparation was purified from unencapsulated drug by Sephadex G-75 gel filtration chromatography, eluting with HEPES-buffered saline (pH 6.5). The drug and phospholipid in the purified preparation were diluted to fall within the linear range or each analytical method (1–50 μg/ml VCR and 5–40 μM phospholipid) and quantitated spectrophotometrically at 298 nm following dissolution in methanol and using a standard phosphate assay [3], respectively. Liposome size was determined by photon correlation spectroscopy using a Coulter N4 Plus particle size analyzer (Beckman Coulter; Fullerton, CA) and reported as the volume-weighted average diameter. The liposome preparations were sterilized by the passage through a 0.2 μm syringe filter, and stored at 4°C until use.
Preparation of anti-HER2 immunoliposomal vincristine
HER2-targeted immunoliposomal vincristine was prepared by co-incubation of vincristine liposomes with the highly internalizable anti-HER2 scFv F5 [35, 40] conjugated at the terminal cysteine to an amphiphilic anchor maleimido-PEG-DSPE essentiallly as described in detail previously [33, 34]. Briefly, F5 having free C-terminal cysteine group was incubated in an aqueous buffer with the solution of maleimido-PEG-DSPE at the cysteine/maleimide molar ratio of 4:1, the excess maleimide groups were capped by reaction with cysteine, and the conjugation product (that forms micelles) was purified from any unreacted protein and excess quencher using gel chromatography. The conjugate was incubated at 60°C with drug-loaded liposomes at a ratio of 15 μg conjugated protein/μmol PL, and quenched on ice for 15 min. Unincorporated F5-PEG-DSPE conjugate (if any) was removed using Sepharose CL-4B gel filtration chromatography eluted with HEPES-buffered saline (pH 6.5). This process has been shown previously to yield approximately 90% incorporation of the F5 targeting moiety into the liposomal formulation and never resulted in a loss of greater than 5% of the liposome-encapsulated VCR or VBL.
Cell culture
Cytotoxicity experiments were performed using the HER-2 overexpressing human mammary carcinoma cell lines SKBR-3 and BT474-M2 (BT474 cells from American Type Culture Collection (Rockville, MD). The BT474-M2 subline was established through in vivo propagation of the original BT474 (ATCC; Manassas, VA) cell line. Briefly, BT474 cells were implanted subcutaneously in NCR nu/nu female mice in the absence of matrigel. Mice that had rapidly growing tumors were sacrificed and sublines established from cells originating from these tumors. Cells from various sublines were reimplanted into groups of 10 NCR nu/nu female mice, and those lines that resulted in greater than 70% tumor take, and uniform tumor growth rates were used in future in vivo studies. The M2 subline was the most effective of the cell lines evaluated, and was used in all subsequent studies.
SKBR-3 cells were grown in McCoy’s 5-A medium supplemented with 10% fetal bovine serum, 0.1 mg/ml streptomycin sulfate, and 100 U/ml Penicillin G. BT474-M2 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 0.1 mg/ml streptomycin sulfate, and 100 U/ml Penicillin G. Cells were exposed to medium containing the indicated drug formulation for 4 h. After exchange with fresh medium, the cells were allowed to incubate at 37°C for 2–3 days. Cell viability was determined by incubation with MTT (0.5 mg/ml) for 2 h, followed by solubilization of the formazan product in acidic isopropanol, and subsequent quantification by absorbance at 540 nm using a microtiter plate reader. IC50 values were calculated using linear regression (Microsoft Excel) between the two drug concentrations bracketing the 50% viability value.
Pharmacokinetic and in vivo drug retention studies
In vivo stability and circulation of the liposomal drug formulation were studied in female Sprague-Dawley rats (190–210 g) with indwelling central venous catheters. The rats were given a single bolus injection (0.5–0.9 ml) of 3H-CHE-labeled Ls-VCR or Ls-VBL at a dose of 5 mg/kg. Blood samples (0.25 ml) were drawn at various times post injection using a heparin-treated syringe and the blood volume was replenished using phosphate buffered physiological saline. The blood samples were diluted with 0.3 ml of ice-cold PBS containing 0.04% EDTA, weighed, and the blood cells were separated by centrifugation for 5 min at 6,000 RPM. The supernatant was collected and assayed for drug by HPLC, and for the liposome 3H-lipid label by scintillation radioactivity counting of 30 μl of the plasma solution. Radioactivity standards were made from the 3H-labeled liposome sample having a known phospholipid concentration and an equal amount of diluted rat plasma was added to account for matrix effects. No dilution was required for the plasma 3H-labeled liposome concentration to fit within the linear range of 0.2–20 nmol phospholipid. The recovery of 3H-labeled liposomal PL from plasma (vs. PBS) is 99.4% with a detection limit of 0.025 nmol phospholipid. Drug analysis was performed by addition of 50 μl of plasma solution to 450 μl of methanol. The samples were then vortexed, cooled to −80°C for a minimum of 2 h. The liquid samples were transferred directly to a centrifuge where they equilibrated to near room temperature while spinning at 13,000 RPM for 10 min. The supernatant was transferred to an auto sampler vial and kept at 4°C until analysis. Plasma drug recovery was determined by extraction of liposomal drugs from spiked plasma as described above. Analysis was conducted on a Dionex HPLC system using a C18 reverse phase silica column (Supelco C-18 column, 250 mm × 4 mm i.d., particle size of 5 μm) preceded by a Supelco C18 guard column. An injection volume of 50 μl was used and the analytes were eluted isocratically at a flow rate of 1.0 ml/min with a mobile phase consisting of 0.21 M aqueous triethylammonium acetate pH 5.5 and acetonitrile (63:37). VCR and VBL are typically eluted in 10.5 min and 15.0 min, respectively, and each was detected by absorbance at 298 nm using a diode array detector. No dilution was required for the plasma VCR/VBL concentration to fit within the linear range of 0.1–10 μg/ml. The linearity of all VCR and VBL stardard curves used here was >0.997. The recovery for VCR and VBL from spiked plasma controls was 100.4 and 102.0%, respectively, and the limit of detection was 0.2 μg/ml for each. Agreement between replicate standard preparations was within 5% and the % relative standard deviation of 5 consecutive injections was <2%. Pharmacokinetic parameters including the tissue half-lives of the drug (t 1/2), clearance (CL), the mean residence time in the tumor (MRT), and the area under the concentration versus time curve (AUC∞) were all determined by non-compartmental pharmacokinetics data analysis using PK Solutions 2.0 software (Summit Research Services; Montrose, CO). Drug release rates from the liposome were characterized by their half-life of release times (T 1/2), and were calculated using the exponential constant (λ), from a single exponential fit to the plot of drug/phospholipid ratio versus post injection time [N(t) = N 0e− λt]. N(t) is the drug-to-PL ratio at time t and N(0) is the same ratio at time 0. The T 1/2 = 0.693/λ.
Antitumor efficacy
BT474-M2 cells were propagated in vitro in RPMI-1640 medium with 10% fetal calf serum, 0.1 mg/mL streptomycin sulfate, and 100 U/ml Penicillin G. NCR nu/nu female mice (5–6 week old; Charles River, Boston, MA) were subcutaneously implanted (at the base of tail) with 60-day sustained-release 0.72-mg 17β-estradiol pellets (Innovative Research of America, Inc., Sarasota, FL), and in 2 days were inoculated subcutaneously with 0.1 ml suspension containing 2 × 107 BT474-M2 cells in cell growth medium containing no additional supplements. The tumor progression was monitored by palpation and caliper measurements of the tumors along the largest (length) and the smallest (width) axis twice a week. The tumor sizes were determined twice weekly from the caliper measurements using the formula: tumor volume = [(length) × (width)2]/2.
At day 20, post tumor cell inoculation, when the tumors reached about 210 mm3 in size (range 144–274 mm3), the mice were randomized into 4 groups of 9 animals/group, and treated by i.v. injection with saline, 1.0 mg/kg of free VCR, 1.0 mg/kg Ls-VCR, or 1.0 mg/kg anti-HER2-ILs-VCR. Each treatment group was administered every 7 days for a total of 3 treatments. General health of the animals was observed by monitoring alertness, grooming, feeding, excreta, skin, fur, mucous membrane conditions, ambulation, breathing, posture, and body weight. Statistical significance of the therapeutic effects for different treatment groups was evaluated using a one-way ANOVA with post hoc Holm-Sidak test (SigmaStat 3.1) of the tumor sizes at the end of the study.
Results
Evaluation of liposomal drug capacity under different loading conditions
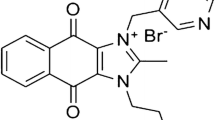
Here we form stable nanoliposomal formulations of vincristine and vinblastine (Fig. 1) using a loading strategy similar to that described by us previously for CPT-11 and the histone deacetylase inhibitor, LAQ824 [8, 11]. The drug is stabilized intraliposomally through complexation of the titratable (cationic) amine groups of VCR or VBL, and a highly charged, non-polymer multivalent anion of sucrose octasulfate (anionic; Fig. 1c), in the liposome interior. The loading of Ls-VCR using this technology is highly efficient for liposomes over a wide range of drug payload (Fig. 2a). The liposomal drug capacity is indicated by the ratio of encapsulated drug to liposomal phospholipid (D/L, g/mol). Between the range of 150–550 g/mol the average loading efficiency was 100.8 ± 5.13% and the resulting drug to liposomal phospholipid ratio correlated well to the input ratio (R 2 = 0.9968). Ls-VBL similarly exhibited efficient of drug loading at 150 g VBL/mol PL (measured efficiency was 102.1 ± 8.1%).
The efficiency of VCR and VBL loading into liposomes. The liposomal drug capacity (g drug/mol phospholipid) as a function of the initial drug/lipid ratio is shown as resulting drug/lipid ratio (open square) and efficiency (filled circle) for VCR (a), or as resulting drug/lipid ratio (open diamond) and efficiency (filled diamond) for VBL (b). Liposomal loading of VCR (filled circle) is also shown as a function of extraliposomal solution pH (c)
The effect of extraliposomal solution pH on the loading efficiency of VCR was also studied. The pH imparts minimal influence on loading between pH 4.5–7.5, but below pH 4.5 the loading was dramatically reduced which may be explained by the decrease in the amount of membrane-permeant neutral (non-protonated) form of the drug due to the shift in the drug acid-base equilibrium at these low pH values. In all subsequent studies, loading was performed at pH 6.5.
In vivo pharmacokinetics and drug retention
The blood circulation characteristics of two liposomal vinca alkaloids, Ls-VCR (Fig. 3a) and Ls-VBL (Fig. 3b), were studied in rats. Ls-VBL demonstrates the advantage of liposomal encapsulation with regard to circulation by exhibiting an AUC of 1,076 μg h/ml which is 2 orders of magnitude greater than that typically observed for unencapsulated VBL [47]. The Ls-VBL formulation was highly stable in circulation as indicated by the encapsulated drug/liposomal phospholipid ratio over time where 73.1% of the VBL remains within the liposome after 48 h in circulation, and the drug release T 1/2 was calculated to be 41.3 h (Fig. 3c). With this liposomal VBL formulation, drug disposition is controlled by the slow release rate of VBL from liposomes in vivo, and thus the drug’s apparent PK parameters are similar to the liposomal PK parameters.
In vivo pharmacokinetic evaluation of the liposomal drug formulation stability and circulation as indicated by % injected dose of the liposomal phospholipid (open square) and VCR (filled square) components of the Ls-VCR formulation (101.6 nm, 104.5 g VCR/mol phospholipid) (a) and liposomal phospholipid (open diamond) and VBL (filled diamond) of the Ls-VBL formulation (99.5 nm, 152.4 g VBL/mol phospholipid) (b). Stability as indicated by retention of the drug within the liposome is shown for Ls-VCR (open square) and Ls-VBL (open diamond) as % original drug/lipid ratio (c)
Evaluation of Ls-VCR (101.6 nm and 104 g VCR/mol PL formulation) shows even greater formulation stability (T 1/2 = 104 h), and reduced systemic clearance (t 1/2 = 18.5 h), compared to the similar composition of Ls-VBL. The blood half-life of the various Ls-VCR formulations ranged from 12.3 to 31.2 h (Table 1). In the group of the three 76.8 nm liposomes and the two 101–102 nm liposomes, the formulations with the lowest drug to lipid ratio (95.1 g/mol) exhibited the longest circulation half-life and mean residence time. This is consistent with lower drug-to-lipid ratios resulting in higher lipid doses being administered, and circulation lifetimes being in part determined by the dose of liposomal carrier. In addition, relatively small liposomes (76.8 nm) retain VCR well, and although not statistically significant, show a trend towards the longest circulation lifetimes of all formulations tested.
In vitro cytotoxicity
As shown previously [35], anti-HER2-immunoliposomes were prepared using anti-HER2 scFv (F5)-PEG-DSPE conjugates to specifically and effectively internalize into HER2-overexpressing cancer cells. Anti-HER2 ILs-VCR also exhibited dramatically improved cytotoxicity in two different HER2-overexpressing breast cancer cell lines (BT474-M2 and SKBR3; Fig. 4). The IC50 for anti-HER2-ILs-VCR is 253-fold lower (p = 6.8e−8)in SKBR-3 cells and 63-fold lower (p = 0.016) in BT474-M2 cells, compared to Ls-VCR. The membrane permeable free VCR exhibits IC50 values of 111 ± 24.4 and 67.3 ± 10.9 nM for SKBR-3 and BT474-M2 cells, respectively, which was in both greater than (p = 9.7e−5 for SKBR-3 and p = 0.021 for BT474-M2) the respective values anti-HER2-ILs-VCR (14.8 ± 1.44 and 29.3 ± 3.06 nM), although in the case of SKBR3 cells the difference was more prominent. These data suggest that prolonged intracellular exposure to the drug from the intracellular reservoir improves the activity. Similar to untreated cells, empty liposomes containing no drug have no affect on cell viability at phospholipid concentrations equal to the highest used here (unpublished data).
In vitro cytotoxic effect of free VCR (open circle), Ls-VCR (open square), and anti-HER2-ILs-VCR (open triangle) on HER2 over-expressing breast cancer cell lines SKBR-3 (a) and BT474-M2 (b). The cellular sensitivity to the VCR formulations is shown as a plot of % viability (compared to untreated cells) as a function of increasing drug concentration. IC50 values (μg/ml) for SKBR-3 cells are: Ls-VCR, 3.04; free VCR, 0.0862; Anti-HER2-ILs-VCR, 0.012; and BT474-M2 cells are: Ls-VCR, 1.53; free VCR, 0.0557; Anti-HER2-ILs-VCR, 0.0243
VCR formulation anti-tumor efficacy against BT474-M2 xenografts in mice
Evaluation of the in vivo anti-tumor efficacy of various VCR formulations was studied in mice with HER2-overexpressing BT474-M2 xenografts (Fig. 5). In this study, we compared saline control, free vincristine, nontargeted Ls-VCR, and anti-HER2-ILs-VCR dosed once weekly for 3 weeks. Growth curves for the average tumor size from each treatment group: saline control, free VCR, Ls-VCR, and anti-HER2-ILs-VCR are displayed in Fig. 5a, with all treatments having a greater therapeutic effect than the saline control. Ls-VCR was similar to, but only slightly more efficacious than free VCR. Anti-HER2-ILs-VCR was the most active of all the tested formulations with five of nine mice showing complete tumor regression, and significant improvements in efficacy. One-way ANOVA with post hoc Holm-Sidak analysis indicates the therapeutic effect of anti-HER2-ILs-VCR was significantly greater than Ls-VCR (p = 0.025) The non-targeted Ls-VCR group had one complete regression, and the saline and free VCR controls had none. The treatment-related toxicity was well within limits of tolerance as none of the treatments caused greater than 10% weight loss (Fig. 5b) or any observable signs of adverse reaction. In fact, following the last (third) treatment the mice in all treatment groups gained weight. There was the weight loss in the saline control the group presumably resulting from the increasing tumor burden.
Tumor size (a–e) and body weight dynamics (f) in nude mice with subcutaneous BT-474-M2 tumor xenografts, treated with various vincristine formulations. All groups containing VCR were administered at a dose of 1 mg/kg. After implantation of BT474-M2 tumor cells animals were treated on days 20, 27, and 34 with saline control (0 cures/9 mice) (open square), free VCR (0 cures/9mice) (filled circle), Ls-VCR (1 cure/9 mice) (open diamond), and anti-HER2-ILs-VCR (5 cures/9 mice) (filled diamond) (a). Error bars indicate the standard error for each group and arrows indicate treatment times. Both Ls-VCR formulations had a liposome size (average ± standard deviation) of 76.8 ± 27.2 nm and a drug/phospholipid ratio of 95.1 g/mol. The dynamics of body weights (b) is shown as the average % weight change compared to the pretreatment level
Discussion
Vinca alkaloids such as vincristine, vinblastine, and vinorelbine are widely used cytotoxic drugs that elicit their effects through disruption of microtubules, resulting in metaphase arrest in dividing cells [41]. Due to their mechanism of action, vinca alkaloids are schedule dependent drugs and thus their activity is affected by the duration of exposure to the drug [17, 18]. As such, vinca alkaloids would benefit from a controlled release dosage form that would effectively prolong the duration of exposure over extended periods of time. Liposomal nanocarriers represent one such dosage form that has been extensively studied for its ability to prolong the pharmacokinetics and subsequent exposure of various drugs, including vinca alkaloids [2, 9, 12].
However, liposome formulations of vinca alkaloids are considerably more difficult to stabilize in vivo when compared to the more widely studied anthracyclines. Possibly, due to the high propensity of doxorubicin molecules for self-association, doxorubicin forms highly stable precipitates inside liposomes following loading using simple pH or ammonium sulfate-gradients [22], and demonstrates release rates on the order of 100 h in vivo [44]. Using similar lipid compositions and gradient-loading strategies allowed achieving the T½ of vincristine leakage from the liposome equal to 17 h [48]. The rapid release from the nanoparticle carrier is unfavorable for the drug’s ability to benefit from the EPR effect in treating solid tumors. Although extravasation efficiency varies depending on tumor location [16] and the various physicochemical properties of the liposomal carrier, including size and surface charge, the maximum accumulation of long-circulating liposomes in tumors has been reported as generally occurring at about 24–48 h post administration [9, 13, 21]. In humans, the circulation lifetimes can even be substantially longer than observed in animal models [27, 49]. Thus, if a substantial proportion of the encapsulated drug is released prior to the liposomes reaching the tumor; the drug is deprived of an advantage of the depot effect whereby the drug is released locally in close proximity to the tumor cells.
The requirement for stable encapsulation is even more important for ligand-targeted formulations, where specific delivery to receptor-overexpressing tumor cells is not possible if the drug is released prematurely before reaching its site of action [36, 42]. The fact that the ligand is not covalently conjugated to the active therapeutic agent can be a considerable advantage over other drug immunoconjugates or immunotoxins that require complicated linker strategies, and where species-dependent differences in linker hydrolysis rates complicate the development of these agents [50]. Controlling the rates of drug release through the nanocarrier’s physicochemical properties and drug encapsulation technology [12], obviates the need for hydrolysis of the chemical linkers. However, perhaps due in part to the relative instability of vincristine liposome formulations, immunotargeted formulations of vincristine have been primarily studied in readily vascularly accessible hematological cancers [43, 44].
A variety of approaches has been advanced to improve in vivo formulation stability, with varying degrees of success depending on the specific vinca alkaloid being delivered. Bally, Mayer, and coworkers successfully substituted sphingomyelin for phosphatidylcholine in cholesterol-containing liposomal vincristine formulations to limit the diffusion of the drug across the membrane, nearly doubling the half-life of vincristine release from 17.1 to 33.3 h [48]. These liposomes, termed “Sphingosomes”, are currently being developed by Hana Biosciences (South San Francisco, CA) for the treatment of non-Hodgkin’s lymphoma and acute lymphoblastic leukemia, and have been studied in both Phase I and II clinical trials [4, 14, 46]. A modification of this approach uses fully hydrogenated sphingomyelin to further stabilize the formulation against in vivo drug leakage, resulting in an increase in circulation lifetime of the sphingosomal vincristine in mice to 13.2 h compared to 9.4 h for the control egg sphingomyelin/cholesterol formulation at the injected lipid dose of 150 μmol PL/kg [19].
High drug-to-lipid ratios can also enhance the formation of intraliposomal drug precipitate, and thus reduce the intraliposome pool of the dissolved, membrane-permeable form of the drug available for transmembrane diffusion, which, along with the permeability constant, determines the drug release rate from the liposomes once in the circulation [12, 20]. The combination of sphingomyelin/cholesterol formulations together with high drug-to-lipid ratios perhaps exceeding the drug solubility product within the liposome can finally give rise to liposomal vincristine with impressive stability (T 1/2 for release of 65 h) [20], however, at the expense of being limited to high drug-to-lipid ratios resulting in corresponding reductions in the lipid dose being administered for a given dose of drug. This may lead to potentially undesirable consequences for the liposome blood clearance, as conventional (non-PEGylated) liposome formulations display dose-dependent pharmacokinetics and thus lower lipid doses result in more rapid clearance via the mononuclear phagocyte system [2, 9, 12]. Although PEGylated liposomes display pharmacokinetics that are less dependent on the administered lipid dose, the further reduction of administered lipid doses of a liposomal carrier necessitated by a combination of a high potency drug with a high drug-to-phospholipid ratio may cause increased clearance of even PEGylated liposomal drugs [12, 23].
While the above-discussed release modification methods [20] did result in slower release rates for vincristine, the results for vinorelbine (T 1/2 = 11.0 h) or vinblastine (T 1/2 = 14.7 h), have not been as successful [45, 51]. This correlates with the increased hydrophobicity of vinblastine and vinorelbine, relative to vincristine [26], and thus their greater membrane permeability. Our approach has been to develop a delivery strategy that can effectively stabilize both the hydrophilic and hydrophobic vinca alkaloids, as well as achieve this stability at low-to-moderate drug-to-lipid ratios where circulation lifetimes will not be compromised. We have previously discovered that a loading strategy that employs a di- or tri-alkylammonium salt of highly sulfated, non-polymeric polyol, sucrose octasulfate, to load and stabilize weakly basic amphipathic drugs intraliposomally resulted in surprising improvements in circulation lifetimes and in vivo drug retention by the carrier [8, 11]. We hypothesize that very high charge density in combination with the multivalent ionic character and compactness of the molecule unachievable with the previously employed polymeric polyanions [52] makes sucrose octasulfate a better agent to immobilize a cationic drug, such as a vinca alkaloid, inside the liposome, while the use of exchangeable substituted ammonium cation with the ionic radius larger than ammonium itself helps to reduce the amount of exchangeable cation immobilized by the non-exchangeable highly charged polyvalent anion and therefore improves the completeness of the drug-for-cation exchange across the liposome membrane in the course of the drug loading. Here, we have applied this technology (which is refered to below as “nanoliposomal formulations” or “nanoliposomes”) to both vincristine and vinblastine (Fig. 1a, b) to see if these vinca alkaloids could be stabilized under a range of formulation conditions.
As described above, we were able to stabilize VCR in liposomes, such that the half life of release was 104.5 h for a 101.6 nm liposome loaded at 104 g VCR/mol PL (~100 g VCR/g lipid; Fig. 3c), which is a 6.7-fold improvement relative to the sphingosomal formulation loaded at the same drug-to-lipid ratio [20], thus demonstrating significant retention of vincristine is possible even in the absence of the highly cohesive lipid compositions that include sphingomyelin and cholesterol. Non-pegylated liposomes typically also display pharmacokinetics that are dependent on size, with smaller sizes being longer circulating [9, 12]. There is concern with drug-loaded liposomes is that the high radius of membrane curvature in smaller liposomes can sometimes cause membrane defects that give rise to increased rates of drug leakage. However, for the smallest VCR formulation (76.8 nm) having the lowest drug to lipid ratio (95.1 g VCR/mol PL), the small size did not appear to have a detrimental effect on circulation lifetimes or in vivo stability.
Although this manuscript focuses on VCR, a single formulation of VBL was also studied to demonstrate that the methodology was not unique to a single vinca alkaloid. The nanoliposomal formulation of vinblastine was also markedly more stable (T 1/2 = 41.3 h at drug-to-lipid ratio of 0.14 g VBL/g lipid) than the previously described sphingosomal formulations at either the high or low drug-to-lipid ratio studied (T 1/2 = 3.1 h for 0.1 g VBL/g lipid and 14.7 h for the 0.3 g VBL/g lipid) [51]. Importantly, for both drugs the stability was high enough to allow liposomes sufficient time to accumulate in solid tumors and thus to take full advantage of the EPR phenomenon and molecular targeting of solid tumors. Additionally, extended circulation lifetimes were maintained over a range of formulation parameters, including at comparatively small sizes (76.8 nm; Table 1), thus suggesting that modifications that may allow for increased extravasation and accumulation in the tumor may also be permissible and desirable for targeted formulations.
Immunoliposomes were prepared through conjugation of an anti-HER2 scFv (F5) to the surface of the liposome via a maleimide-activated PEG-DSPE anchor [33, 34]. We have previously demonstrated that antibody fragments capable of inducing internalization upon binding to the tyrosine kinase receptors, EGFR and HER2/neu, were able to improve the antitumor activity of liposomally encapsulated anticancer drugs [29, 35, 37]. HER2-specific targeting of nanoliposomal VCR in vitro not only restored the liposomal drug efficiency to the level of the free drug, but also, surprisingly, surpassed it, giving evidence that despite effective stabilization against drug leakage, intraliposomal sucroseoctasulfate afforded sufficient bioavailability of the encapsulated VCR. Targeted antitumor efficacy for F5-immunoliposomal vincristine was demonstrated also in vivo in a HER2-overexpressing breast cancer model (Fig. 5). Efficacy was significantly improved for the HER2-targeted nanoliposomal vincristine when compared to nontargeted nanoliposomal vincristine (p = 0.025) demonstrating the sufficient stabilization of encapsulation can result in targeted antitumor activity in solid tumors. Vincristine is used clinically in the treatment of acute leukemia, non-Hodgkin’s malignant lymphomas, Hodgkin’s disease, neuroblastomas, rhabdosarcomas, and Wilm’s tumors [41]. It has also been clinically in the treatment of melanomas [24, 25] and small cell lung cancer [6]. Vincristine is not used widely in the treatment of breast cancer, although vinorelbine is used in third line treatment. However, changes in the pharmacokinetics, bioavailability, and tumor exposure of the drug resulting from stable liposome encapsulation, as well as the ability to be molecularly targeted may alter the range of cancers susceptible to treatment with vincristine, which has a relatively nonspecific mechanism of action. Alternatively, this proof-of-concept study suggests that for the first time vincristine may be targeted for the treatment of solid tumors, and that similar formulations using different antibodies targeted to melanoma, neuroblastomas, or small cell lung cancer now have the potential for being efficacious in treating these cancers.
Abbreviations
- Chol:
-
Cholesterol
- 3H-CHE:
-
Tritiated cholesterylhexadecylether
- CLs:
-
Conventional liposomes
- ILs:
-
Immunoliposomes
- i.v.:
-
Intravenous
- DSPC:
-
1,2-Distearoyl-sn-glycero-3-phosphocholine
- Ls-VCR:
-
Liposomal vincristine
- PEG-DSPE:
-
N-(Polyethyleneglycol)distearoylphosphatidylethanolamine
- PL:
-
Phospholipid
- SSL:
-
Sterically stabilized liposomes
- TEA:
-
Triethylammonium
- TEA8SOS:
-
Triethylammonium sucroseoctasulfate
- VCR:
-
Vincristine
References
Abraham SA, Edwards K, Karlsson G, MacIntosh S, Mayer LD, McKenzie C, Bally MB (2002) Formation of transition metal-doxorubicin complexes inside liposomes. Biochim Biophys Acta 1565:41–54
Allen TM, Cheng WW, Hare JI, Laginha KM (2006) Pharmacokinetics and pharmacodynamics of lipidic nano-particles in cancer. Anticancer Agents Med Chem 6:513–523
Bartlett GR (1959) Phosphorous assay in column chromatography. J Biol Chem 234:466–468
Bedikian AY, Vardeleon A, Smith T, Campbell S, Namdari R (2006) Pharmacokinetics and urinary excretion of vincristine sulfate liposomes injection in metastatic melanoma patients. J Clin Pharmacol 46:727–737
Boehlke L, Winter JN (2006) Sphingomyelin/cholesterol liposomal vincristine: a new formulation for an old drug. Expert Opin Biol Ther 6:409–415
Cheng S, Evans WK, Stys-Norman D, Shepherd FA (2007) Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2:348–354
Cullis PR, Hope MJ, Bally MB, Madden TD, Mayer LD, Fenske DB (1997) Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim Biophys Acta 1331:187–211
Drummond DC, Marx C, Guo Z, Scott G, Noble C, Wang D, Pallavicini M, Kirpotin DB, Benz CC (2005) Enhanced pharmacodynamic and antitumor properties of a histone deacetylase inhibitor encapsulated in liposomes or ErbB2-targeted immunoliposomes. Clin Cancer Res 11:3392–3401
Drummond DC, Meyer OM, Hong K, Kirpotin DB, Papahadjopoulos D (1999) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 51:691–743
Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB (2008) Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci 97:4696–4740
Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB (2006) Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res 66:3271–3277
Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB (2008) Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci 97:4696–4740
Gabizon A, Chemla M, Tzemach D, Horowitz AT, Goren D (1996) Liposome longevity and stability in circulation: effects on the in vivo delivery to tumors and therapeutic efficacy of encapsulated anthracyclines. J Drug Target 3:391–398
Gelmon KA, Tolcher A, Diab AR, Bally MB, Embree L, Hudon N, Dedhar C, Ayers D, Eisen A, Melosky B, Burge C, Logan P, Mayer LD (1999) Phase I study of liposomal vincristine. J Clin Oncol 17:697–705
Haran G, Cohen R, Bar LK, Barenholz Y (1993) Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphiphathic weak bases. Biochim Biophys Acta 1151:201–215
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 95:4607–4612
Horton JK, Houghton PJ, Houghton JA (1988) Relationships between tumor responsiveness, vincristine pharmacokinetics and arrest of mitosis in human tumor xenografts. Biochem Pharmacol 37:3995–4000
Jackson DV Jr, Bender RA (1979) Cytotoxic thresholds of vincristine in a murine and a human leukemia cell line in vitro. Cancer Res 39:4346–4349
Johnston MJ, Semple SC, Klimuk SK, Ansell S, Maurer N, Cullis PR (2007) Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta 1768:1121–1127
Johnston MJ, Semple SC, Klimuk SK, Edwards K, Eisenhardt ML, Leng EC, Karlsson G, Yanko D, Cullis PR (2006) Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta 1758:55–64
Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW (2006) Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 66:6732–6740
Lasic DD, Frederick PM, Stuart MCA, Barenholz Y, McIntosh TJ (1992) Gelation of liposome interior: a novel method for drug encapsulation. FEBS Lett 312:255–258
Laverman P, Brouwers AH, Dams ETM, Oyen WJG, Storm G, Van Rooigen N, Corstens FHM, Boerman OC (2000) Preclinical and clincal evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose. J Pharmacol Exp Ther 293:996–1001
Legha SS (1997) Durable complete responses in metastatic melanoma treated with interleukin-2 in combination with interferon alpha and chemotherapy. Semin Oncol 24:S39–S43
Lens MB, Eisen TG (2003) Systemic chemotherapy in the treatment of malignant melanoma. Expert Opin Pharmacother 4:2205–2211
Lobert S, Fahy J, Hill BT, Duflos A, Etievant C, Correia JJ (2000) Vinca alkaloid-induced tubulin spiral formation correlates with cytotoxicity in the leukemic L1210 cell line. Biochemistry 39:12053–12062
Lyass O, Uziely B, Ben-Yosef R, Tzemach D, Heshing NI, Lotem M, Brufman G, Gabizon A (2000) Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer 89:1037–1047
Maeda H, Matsumura Y (1989) Tumoritropic and lymphotropic principles of macromolecular drugs. CRC Crit Rev Therap Drug Carrier System 6:193–210
Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, Kirpotin DB, Park JW (2005) Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res 65:11631–11638
Mayer LD, Bally MB, Hope MJ, Cullis PR (1985) Uptake of antineoplastic agents into large unilamellar vesicles in response to a membrane potential. Biochim Biophys Acta 816:294–302
Mayer LD, Bally MB, Loughrey H, Masin D, Cullis PR (1990) Liposomal vincristine preparations which exhibit decreased drug toxicity and increased activity against murine L1210 and P388 tumors. Cancer Res 50:575–579
Mayer LD, Nayar R, Thies RL, Boman NL, Cullis PR, Bally MB (1993) Identification of vesicle properties that enhance the antitumor activity of liposomal vincristine against murine L1210 leukemia. Cancer Chemother Pharmacol 33:17–24
Nellis DF, Ekstrom DL, Kirpotin DB, Zhu J, Andersson R, Broadt TL, Ouellette TF, Perkins SC, Roach JM, Drummond DC, Hong K, Marks JD, Park JW, Giardina SL (2005) Preclinical manufacture of an anti-HER2 scFv-PEG-DSPE, liposome-inserting conjugate. 1. Gram-scale production and purification. Biotechnol Prog 21:205–220
Nellis DF, Giardina SL, Janini GM, Shenoy SR, Marks JD, Tsai R, Drummond DC, Hong K, Park JW, Ouellette TF, Perkins SC, Kirpotin DB (2005) Preclinical manufacture of anti-HER2 liposome-inserting, scFv-PEG-lipid conjugate. 2. Conjugate micelle identity, purity, stability, and potency analysis. Biotechnol Prog 21:221–232
Nielsen UB, Kirpotin DB, Pickering EM, Hong K, Park JW, Refaat Shalaby M, Shao Y, Benz CC, Marks JD (2002) Therapeutic efficacy of anti-ErbB2 immunoliposomes targeted by a phage antibody selected for cellular endocytosis. Biochim Biophys Acta 1591:109–118
Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC (2004) Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets 8:335–353
Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC (2002) Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res 8:1172–1181
Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D (2008) High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol 9:247–256
Pinkerton CR, Blanc Vincent MP, Bergeron C, Fervers B, Philip T (2000) Induction chemotherapy in metastatic neuroblastoma–does dose influence response? A critical review of published data standards, options and recommendations (SOR) project of the National Federation of French Cancer Centres (FNCLCC). Eur J Cancer 36:1808–1815
Poul MA, Becerril B, Nielsen UB, Morisson P, Marks JD (2000) Selection of tumor-specific internalizing human antibodies from phage libraries. J Mol Biol 301:1149–1161
Rowinsky E, Donehower R (1996) Antimicrotubule Agents. In: Chabner B, Longo D (eds) Cancer chemotherapy and biotherapy: principles and practice. Lippincott-Raven Publishers, Philadelphia, pp 263–296
Sapra P, Allen TM (2003) Ligand-targeted liposomal anticancer drugs. Prog Lipid Res 42:439–462
Sapra P, Allen TM (2004) Improved outcome when B-cell lymphoma is treated with combinations of immunoliposomal anticancer drugs targeted to both the CD19 and CD20 epitopes. Clin Cancer Res 10:2530–2537
Sapra P, Moase EH, Ma J, Allen TM (2004) Improved therapeutic responses in a xenograft model of human B lymphoma (Namalwa) for liposomal vincristine versus liposomal doxorubicin targeted via anti-CD19 IgG2a or Fab′ fragments. Clin Cancer Res 10:1100–1111
Semple SC, Leone R, Wang J, Leng EC, Klimuk SK, Eisenhardt ML, Yuan ZN, Edwards K, Maurer N, Hope MJ, Cullis PR, Ahkong QF (2005) Optimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activity. J Pharm Sci 94:1024–1038
Thomas DA, Sarris AH, Cortes J, Faderl S, O’Brien S, Giles FJ, Garcia-Manero G, Rodriguez MA, Cabanillas F, Kantarjian H (2006) Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer 106:120–127
van Asperen J, Schinkel AH, Beijnen JH, Nooijen WJ, Borst P, van Tellingen O (1996) Altered pharmacokinetics of vinblastine in Mdr1a P-glycoprotein-deficient Mice. J Natl Cancer Inst 88:994–999
Webb MS, Harasym TO, Masin D, Bally MB, Mayer LD (1995) Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumour models. Br J Cancer 72:896–904
Working PK, Dayan AD (1996) Pharmacological-toxicological expert report—Caelyx(TM) (Stealth(R) liposomal doxorubicin HCl)—Foreword. Hum Exp Toxicol 15:751–785
Wu AM, Senter PD (2005) Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 23:1137–1146
Zhigaltsev IV, Maurer N, Akhong QF, Leone R, Leng E, Wang J, Semple SC, Cullis PR (2005) Liposome-encapsulated vincristine, vinblastine and vinorelbine: a comparative study of drug loading and retention. J Control Release 104:103–111
Zhu G, Oto E, Vaage J, Quinn Y, Newman M, Engbers C, Uster P (1996) The effect of vincristine-polyanion complexes in STEALTH liposomes on pharmacokinetics, toxicity and anti tumor activity. Cancer Chemother Pharmacol 39:138–142
Acknowledgments
D. C. Drummond was supported in part by a New Investigator Award from the California Breast Cancer Research Program of the University of California, Grant Number 7KB-0066.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Noble, C.O., Guo, Z., Hayes, M.E. et al. Characterization of highly stable liposomal and immunoliposomal formulations of vincristine and vinblastine. Cancer Chemother Pharmacol 64, 741–751 (2009). https://doi.org/10.1007/s00280-008-0923-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0923-3