Abstract
Purpose
Potential clinical use of ultrasound (US) in enhancing the effects of anticancer drugs in the treatment of cancers has been highlighted in previous reports. Increased uptake of drugs by the cancer cells due to US has been suggested as a mechanism. However, the precise mechanism of the enhancement has not yet been elucidated. Here, the combined effects of low-intensity pulsed US and doxorubicin (DOX) on cell killing and apoptosis induction of U937 cells, and mechanisms involved were investigated.
Methods
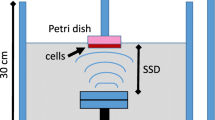
Human myelomonocytic lymphoma U937 cells were used for the experiments. Experiments were conducted in 4 groups: (1) non-treated, (2) DOX treated (DOX), (3) US treated (US), and (4) combined (DOX + US). In DOX +US, cells were exposed to 5 μM DOX for 30 min and sonicated by 1 MHz pulsed US (PRF 100 Hz, DF 10%) at intensities of 0.2–0.5 W/cm2 for 60 s. The cells were washed and incubated for 6 h. The viability was evaluated by Trypan blue dye exclusion test and apoptosis and incorporation of DOX was assessed by flow cytometry. Involvement of sonoporation in molecular incorporation was evaluated using FITC-dextran, hydroxyl radical formation was measured by electron paramagnetic resonance-spin trapping, membrane alteration including lipid peroxidation and membrane fluidity by DOX was evaluated using cis-parinaric acid and perylene fluorescence polarization method, respectively.
Results
Synergistic enhancement in cell killing and additive enhancement in induction of apoptosis were observed at and above 0.3 W/cm2. No enhancement was observed at 0.2 W/cm2 in cell killing and induction of apoptosis. Hydroxyl radicals formation was detected at and above 0.3 W/cm2. The radicals were produced more in the DOX + US than US alone. Incorporation of DOX was increased 13% in DOX + US (vs. DOX) at 0.5 W/cm2. Involvement of sonoporation for increase of drug uptake was suggested by experiment using FITC-labeled dextran. We made the hypothesis that DOX treatment made the cells weaken against the mechanical effect of the US. Although treatment of DOX at 5 μM for 30 min did not affect lipid peroxidation and fluidity of cell membrane significantly, higher concentration and longer treatment of DOX induced the significant alteration of cell membrane.
Conclusion
Mechanisms of enhancements could be (1) increase in incorporation of the DOX by US involved with sonoporation, (2) enhancement of the cavitation by DOX. Cavitation is required for the enhancement of the effect of DOX. Although the precise involvement of the membrane modifications by DOX in the enhancement remains to be elucidated, they could be involved in the latent effects.





Similar content being viewed by others
References
Awato S, Kinoshita K, Ikegami A (1977) Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry 16:2319–2324
Bachur NR, Gordon SL, Gee MV (1977) Anthracycline antibiotic augmentation of microsomal electron transport and free radical formation. Mol Pharmacol 13:901–910
Bachur NR, Yu F, Johnson R, Hickey R, Wu Y, Malkas L (1992) Helicase inhibition by anthracycline anticancer agents. Mol Pharmacol 41:993–998
Bao S, Thrall BD, Miller DL (1997) Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol 23:953–959
Benchekroun MN, Robert J (1992) Measurement of doxorubicin-induced lipid peroxidation under the conditions that determine cytotoxicity in cultured tumor cells. Anal Biochem 201:326–330
Capranico G, Kohn KW, Pommier Y (1990) Local sequence requirements for DNA cleavage by mammalian topoisomerase II in the presence of doxorubicin. Nucleic Acids Res 25:6611–6619
Fechheimer M, Boylan JF, Parker S, Sisken JE, Patel GL, Zimmer SG (1987) Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc Natl Acad Sci USA 84:8463–8467
Feril LB Jr, Kondo T (2005) Major factors involved in the inhibition of ultrasound-induced free radical production and cell killing by pre-sonication incubation or by high cell density. Ultrason Sonochem 12:353–357
Feril LB Jr, Kondo T, Cui ZG, Tabuchi Y, Zhao QL, Ando H, Misaki T, Yoshikawa H, Umemura S (2005) Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett 221:145–152
Feril LB Jr, Kondo T, Zhao QL, Ogawa R (2002) Enhancement of hyperthermia-induced apoptosis by non-thermal effects of ultrasound. Cancer Lett 178:63–70
Feril LB Jr, Kondo T, Zhao QL, Ogawa R, Tachibana K, Kudo N, Fujimoto S, Nakamura S (2003) Enhancement of ultrasound-induced apoptosis and cell lysis by echo-contrast agents. Ultrasound Med Biol 29:331–337
Feril LB, Kondo T, Umemura S, Tachibana K, Manalo AH, Riesz P (2002) Sound waves and antineoplastic drugs: the possibility of an enhanced combined anticancer therapy. J Med Ultrasonics 29
Harrison GH, Balcer-Kubiczek EK, Eddy HA (1991) Potentiation of chemotherapy by low-level ultrasound. Int J Radiat Biol 59:1453–1466
Harrison GH, Balcer-Kubiczek EK, Gutierrez PL (1996) In vitro action of continuous-wave ultrasound combined with adriamycin, X rays or hyperthermia. Radiat Res 145:98–101
Harrison GH, Balcer-Kubiczek EK, Gutierrez PL (1996) In vitro mechanisms of chemopotentiation by tone-burst ultrasound. Ultrasound Med Biol 22:355–362
Hedley D, Chow S (1992) Flow cytometric measurement of lipid peroxidation in vital cells using parinaric acid. Cytometry 13:686–692
Hill CR (1967) Changes in tissue permeability produced by ultrasound. Br J Radiol 40:317–318
Honda H, Kondo T, Zhao QL, Feril LB Jr, Kitagawa H (2004) Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med Biol 30:683–692
Jedrzejczak M, Koceva-Chyla A, Gwozdzinski K, Jozwiak Z (1999) Changes in plasma membrane fluidity of immortal rodent cells induced by anticancer drugs doxorubicin, aclarubicin and mitoxantrone. Cell Biol Int 23:497–506
Kagiya G, Ogawa R, Tabuchi Y, Feril LB Jr, Nozaki T, Fukuda S, Yamamoto K, Kondo T (2006) Expression of heme oxygenase-1 due to intracellular reactive oxygen species induced by ultrasound. Ultrason Sonochem 13:388–396
Kremkau FW (1979) Cancer therapy with ultrasound: a historical review. J Clin Ultrasound 7:287–300
Kuypers FA, van den Berg JJ, Schalkwijk C, Roelofsen B, Op den Kamp JA (1987) Parinaric acid as a sensitive fluorescent probe for the determination of lipid peroxidation. Biochim Biophys Acta 921:266–274
Loverock P, ter Haar G, Ormerod MG, Imrie PR (1990) The effect of ultrasound on the cytotoxicity of adriamycin. Br J Radiol 63:542–546
Miller DL, Williams AR, Morris JE, Chrisler WB (1998) Sonoporation of erythrocytes by lithotripter shockwaves in vitro. Ultrasonics 36:947–952
Murphree SA, Tritton TR, Smith PL, Sartorelli AC (1981) Adriamycin-induced changes in the surface membrane of sarcoma 180 ascites cells. Biochim Biophys Acta 649:317–324
Nozaki T, Ogawa R, Feril LB Jr, Kagiya G, Fuse H, Kondo T (2003) Enhancement of ultrasound-mediated gene transfection by membrane modification. J Gene Med 5:1046–1055
Ogawa R, Kagiya G, Feril LB Jr, Nakaya N, Nozaki T, Fuse H, Kondo T (2004) Ultrasound mediated intravesical transfection enhanced by treatment with lidocaine or heat. J Urol 172:1469–1473
Pagnini U, Pacilio C, Florio S, Crispino A, Claudio PP, Giordano A, Pagnini G (2000) Medroxyprogesterone acetate increases anthracyclines uptake in chronic lymphatic leukemia cells: role of nitric oxide and lipid peroxidation. Anticancer Res 20:33–42
Rosenthal I, Sostaric JZ, Riesz P (2004) Sonodynamic therapy- a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem 11:349–363
Saad AH, Hahn GM (1989) Ultrasound enhanced drug toxicity on Chinese hamster ovary cells in vitro. Cancer Res 49:5931–5934
Saad AH, Hahn GM (1992) Ultrasound-enhanced effects of adriamycin against murine tumors. Ultrasound Med Biol 18:715–723
Tata DB, Biglow J, Wu J, Tritton TR, Dunn F (1996) Ultrasound-enhanced hydroxyl radical production from two clinically employed anticancer drugs, adriamycin and mitomycin C. Ultrasonics Sonochem 3:39–45
Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF (1984) Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 226:466–468
Umemura S, Yumita N, Okano Y, Kaneuchi M, Magario N, Ishizaki M, Shimizu K, Sano Y, Umemura K, Nishigaki R (1997) Sonodynamically induced in vitro cell damage enhanced by adriamycin. Cancer Lett 121:195–201
Yu T, Bai J, Hu K, Wang Z (2003) The effect of free radical scavenger and antioxidant on the increase in intracellular adriamycin accumulation induced by ultrasound. Ultrason Sonochem 10:33–35
Yu T, Wang Z, Jiang S (2001) Potentiation of cytotoxicity of adriamycin on human ovarian carcinoma cell line 3AO by low-level ultrasound. Ultrasonics 39:307–309
Acknowledgments
This study was in a part supported by the Research and Development Committee Program of the Japan Society of Ultrasonics in Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, T., Kondo, T., Ogawa, R. et al. Combination of doxorubicin and low-intensity ultrasound causes a synergistic enhancement in cell killing and an additive enhancement in apoptosis induction in human lymphoma U937 cells. Cancer Chemother Pharmacol 61, 559–567 (2008). https://doi.org/10.1007/s00280-007-0503-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0503-y