Abstract
Purpose
We compared the safety, toxicity, biocompatibility and anti-tumour efficacy of a novel chitosan-egg phosphatidylcholine (ePC) implantable drug delivery system that provides controlled and sustained release of paclitaxel (PTXePC) versus commercial paclitaxel formulated in Cremophor EL (PTXCrEL).
Methods
Toxicity studies were conducted in healthy CD-1 female mice, whereas efficacy studies were performed in the SKOV-3 xenograft model of ovarian cancer. Treatments consisted of intraperitoneal (IP) implantation of drug-free or PTXePC formulations, IP bolus PTXCrEL, or Cremophor EL (CrEL) vehicle. Toxicity was assessed as number of deaths, weight loss, serum hepatic enzyme levels and histopathological changes.
Results
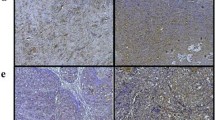
Mice implanted with drug-free or PTXePC formulations did not exhibit observable toxicities, local inflammation or fibrous encapsulation of the implant. In contrast, mice receiving PTXCrEL or CrEL encountered significant toxicity, lethality, abnormal peritoneal organ morphology and hepatic inflammation. The maximum tolerable dose (MTD) of PTXCrEL was 20 mg/kg/week, whereas PTX doses of up to 280 mg/kg/week were well tolerated when administered as PTXePC. Enhanced anti-tumour efficacy was achieved with PTXePC in contrast to PTXCrEL with the same total dose of 60 mg/kg PTX.
Conclusions
The novel PTXePC formulation is a safer and better tolerated method for PTX administration, with significant increase in MTD and enhanced anti-tumour efficacy, suggesting improved therapeutic index with possible clinical implications in the treatment of ovarian tumours.




Similar content being viewed by others
References
Armstrong D, Bundy B, Wenzel L, Huang H, Baergen R, Lele S, Copeland L, Walker J, Burger R, Group GO (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34-43
Dedrick RL, Myers CE, Bungay PM, DeVita VTJ (1978) Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 62:1–11
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794–7803
Grant J, Blicker M, Piquette-Miller M, Allen C (2005) Hybrid films from blends of chitosan and egg phosphatidylcholine for localized delivery of paclitaxel. J Pharm Sci 94:1512–1527
Ho EA, Vassileva V, Allen C, Piquette-Miller M (2005) In vitro and in vivo characterization of a novel biocompatible polymer-lipid implant system for the sustained delivery of paclitaxel. J Control Release 104:181–191
Jang SH, Wientjes MG, Au JL (2001) Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Invest New Drugs 19:113–123
Le Garrec D, Gori S, Luo L, Lessard D, Smith DC, Yessine MA, Ranger M, Leroux JC (2004) Poly(N-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J Control Release 99:83–101
Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JVr (2006) Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol 100:437–438
Mori T, Kinoshita Y, Watanabe A, Yamaguchi T, Hosokawa K, Honjo H (2006) Retention of paclitaxel in cancer cells for 1 week in vivo and in vitro. Cancer Chemother Pharmacol (Epub ahead of print)
Nicoletti MI, Lucchini V, D’Incalci M, Giavazzi R (1994) Comparison of paclitaxel and docetaxel activity on human ovarian carcinoma xenografts. Eur J Cancer 30A:691–696
O’Shaughnessy JA, Fisherman JS, Cowan KH (1994) Combination paclitaxel (Taxol) and doxorubicin therapy for metastatic breast cancer. Semin Oncol 21:19–23
Polizzi D, Pratesi G, Tortoreto M, Supino R, Riva A, Bombardelli E, Zunino F (1999) A novel taxane with improved tolerability and therapeutic activity in a panel of human tumor xenografts. Cancer Res 59:1036–1040
Rowinsky EK, Donehower RC (1995) Paclitaxel (taxol). N Engl J Med 332:1004–1014
Sharma D, Chelvi TP, Kaur J, Chakravorty K, De TK, Maitra A, Ralhan R (1996) Novel Taxol formulation: polyvinylpyrrolidone nanoparticle-encapsulated Taxol for drug delivery in cancer therapy. Oncol Res 8:281–286
Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH (1996) Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res 56:2112–2115
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589
Vassileva V, Allen C, Piquette-Miller M (2004) Release profile characterization of a novel implantable paclitaxel loaded lipid polymer system in cd-1 mice. AAPS Journal 6
Acknowledgments
The authors would like to thank Mr. Ji Zhang for his technical assistance. This work was supported by grants from the Ontario Cancer Reseach Network and the National Cancer Institute of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vassileva, V., Grant, J., De Souza, R. et al. Novel biocompatible intraperitoneal drug delivery system increases tolerability and therapeutic efficacy of paclitaxel in a human ovarian cancer xenograft model. Cancer Chemother Pharmacol 60, 907–914 (2007). https://doi.org/10.1007/s00280-007-0449-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0449-0