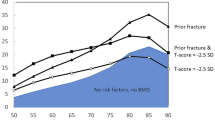
Abstract
We report the first evaluation of bone quality in 70 thalassemia intermedia (TI) patients (37 males, 33 females, age 41 ± 12 years). Thirty-three patients (47%) had been transfused, 34 (49%) had been splenectomized, 39 (56%) were on iron chelation therapy, and 11 (16%) were on hydroxyurea. Mean hemoglobin was 9.2 ± 1.5 g/dl, median ferritin 537 ng/dl (range 14–4893), and mean liver iron concentration 7.6 ± 6.4 mg Fe/g dw. Fifteen patients (21%) had endocrinopathies, and 29 (41%) had vitamin D deficiency. Bone quantity (bone mineral density, BMD) and bone quality (trabecular bone score, TBS) were evaluated by densitometry. In 53/70 patients (76%), osteopathy was found (osteoporosis in 26/53, osteopenia in 27/53). BMD values were higher in the never-transfused patients and in the not-chelated group. A highly significant correlation was found between splenectomy and BMD at all the sites, with lower values in the splenectomized patients. TBS values were significantly lower in TI patients than in 65 non-thalassemic controls (1.22 vs 1.36, p < 0.01), mainly in those splenectomized and in the transfused and chelated groups (p < 0.01). TBS did not correlate with liver iron concentration values. Our data disclose the major role of non-invasive bone quality evaluation in TI patients, especially those with the worst health state, to obtain a comprehensive assessment of fracture risk. Splenectomy seems to play a major part in bone complications.




Similar content being viewed by others
References
Musallam KM, Rivella S, Vichinsky E, Rachmilewitz EA (2013) Non-transfusion-dependent thalassemias. Haematologica 98:833–844
Weatherall DJ (2012) The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev 26:S3–S6
Weatherall DJ (2001) Phenotype-genotype relationships in monogenic disease: lessons from the thalassemias. Nat Rev Genet 2:245–255
Taher AT, Musallam KM, El-Beshlawy A et al (2010) Age-related complications in treatment-naïve patients with thalassemia intermedia. Br J Haematol 150:486–489
Inati A, Noureldine MA, Mansour A, Abbas HA (2015) Endocrine and bone complications in β-thalassemia intermedia: current understanding and treatment. Biomed Res Int 813098
Kanis JA on behalf of the World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary health care level. Technical report. World Health Organization Collaborating Center for Metabolic Bone Diseases. University of Sheffield, UK 12
Kanis J, McCloskey EV, Johansson H et al (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Rubin CD (2005) Emerging concepts in osteoporosis and bone strength. Curr Med Res Opin 21:1049–1056
Chatterjee R, Shah FT, Davis BA, Byers M et al (2012) Prospective study of histomorphometry, biochemical bone markers and bone densitometric response to pamidronate in β-thalassemia presenting with osteopenia-osteoporosis syndrome. Br J Haematol 159:462–471
Mahachoklertwattana P, Chuansumrit A, Sirisriro R et al (2003) Bone mineral density, biochemical and hormonal profiles in suboptimally treated children and adolescents with beta-thalassemia disease. Clin Endocrinol 58:273–279
Bousson V, Bergot C, Sutter B, Levitz P, Cortet B (2012) Scientific Committee of the Groupe de Recherche et d’Information sur les Ostéoporoses trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int 23:1489–1501
Silva BC, Leslie WD, Resch H et al (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Ulivieri FM, Silva BC, Sardanelli F et al (2014) Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 47:435–448
Baldini M, Marcon A, Cassin R et al (2014) Beta-Thalassaemia intermedia: evaluation of endocrine and bone complications. BioMed Res Int. Article ID 174581
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg M-A (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14:302–312
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a non-invasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42:775–787
Winzenrieth R, Michelet F, Hans D (2012) Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: effects of resolution and noise. J Clin Densitom 16:287–296
Roux JP, Wegrzyn J, Boutroy S, Bouxsein ML, Hans D, Chapurlat R (2013) The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: an ex vivo study. Osteoporos Int 24:2455–2460
Siris ES, Brenneman SK, Barrett-Connor E et al (2006) The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50-99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 17:565–574
Leslie WD, Johansson H, Kanis JA et al (2014) Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 25:2271–2277
McCloskey EV, Oden A, Harvey NC et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31:940–948
McCloskey EV, Oden A, Harvey NC et al (2015) Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96:500–509
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769
Baldini M, Ulivieri FM, Forti S et al (2014) Spine bone texture assessed by trabecular bone score (TBS) to evaluate bone health in thalassemia major. Calcif Tissue Int 95:540–546
Boutroy S, Hans D, Sornay-Rendu E et al (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Vogiatzi MG, Macklin EA, Fung EB et al (2006) Prevalence of fractures among the thalassemia syndromes in North America. Bone 38:571–575
Baldini M, Forti S, Marcon A et al (2010) Endocrine and bone disease in appropriately treated adult patients with beta-thalassemia major. Ann Hematol 89:1207–1213
De Sanctis V, Eleftheriou A, Malaventura C (2004) Thalassemia international federation study group on growth and endocrine complications in thalassemia prevalence of endocrine complications and short stature in patients with thalassemia major: a multicenter study by the thalassemia international federation (TIF). Pediatr Endocrinol Rev 2(Suppl 2):249–255
Martinez-Jabaloyas JM (2013) Hypogonadism global epidemiology and transversal relationships. Arch Esp Urol 66(7):632–638
Almandoz JP, Gharib H (2012) Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR et al (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
Taher AT, Porter JB, Viprakasit V et al (2013) Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassemia (NTDT) patients: 1-year extension results from the THALASSA study. Ann Hematol 92(11):1485–1493
Acknowledgements
We thank Prof. Cass Ingerson for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The study was partially funded by grants of Ministero della Salute (RC 2016).
Ethical approval
All the procedures performed were in accordance with the ethical standards of the responsible research committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study has been approved by the institutional research ethics committee. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Baldini, M., Marcon, A., Ulivieri, F.M. et al. Bone quality in beta-thalassemia intermedia: relationships with bone quantity and endocrine and hematologic variables. Ann Hematol 96, 995–1003 (2017). https://doi.org/10.1007/s00277-017-2959-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2959-0