Abstract
Clinical resistance to chemotherapy in acute myeloid leukemia (AML) is associated with the expression of the multidrug resistance (MDR) proteins P-glycoprotein, encoded by the MDR1/ABCB1 gene, multidrug resistant-related protein (MRP/ABCC1), the lung resistance-related protein (LRP), or major vault protein (MVP), and the breast cancer resistance protein (BCRP/ABCG2). The clinical value of MDR1, MRP1, LRP/MVP, and BCRP messenger RNA (mRNA) expression was prospectively studied in 154 newly diagnosed AML patients ≥60 years who were treated in a multicenter, randomized phase 3 trial. Expression of MDR1 and BCRP showed a negative whereas MRP1 and LRP showed a positive correlation with high white blood cell count (respectively, p < 0.05, p < 0.001, p < 0.001 and p < 0.001). Higher BCRP mRNA was associated with secondary AML (p < 0.05). MDR1 and BCRP mRNA were highly significantly associated (p < 0.001), as were MRP1 and LRP mRNA (p < 0.001) expression. Univariate regression analyses revealed that CD34 expression, increasing MDR1 mRNA as well as MDR1/BCRP coexpression, were associated with a lower complete response (CR) rate and with worse event-free survival and overall survival. When adjusted for other prognostic actors, only CD34-related MDR1/BCRP coexpression remained significantly associated with a lower CR rate (p = 0.03), thereby identifying a clinically resistant subgroup of elderly AML patients.
Similar content being viewed by others
Introduction
Clinical resistance to chemotherapy in acute myeloid leukemia (AML) is often associated with the expression of (membrane) transport-associated multidrug resistance (MDR) proteins. Expression of P-glycoprotein (P-gp), encoded by the MDR1 gene, is an independent adverse prognostic factor for response and survival in de novo AML [1–4]. Moreover, it has been shown that besides P-gp, also the MDR-related protein (MRP1/ABCC1) and the lung resistance-related protein (LRP), also designated as the major vault protein (MVP), are expressed in AML. However, the prognostic significance of the latter resistance proteins has not been settled [3, 5–7]. Some years ago, a new drug resistant protein, i.e., the breast cancer resistance protein (BCRP/ABCG2), which is the equivalent of the mitoxantrone (MXT) resistant protein and the placental ABC transporter (ABCP), was found to be expressed in AML [8–13]. The precise role of either resistance proteins among poor risk AML such as in patients of older age has not been established. This study prospectively investigated the relevance of MDR1, MRP1, LRP, and BCRP messenger RNA (mRNA) expression in combination with known prognostic characteristics like CD34 expression, white blood cell (WBC) count, and secondary AML as possible denominators of response and survival in patients with AML aged 60+ who were treated in the same clinical trial.
Patients and methods
Patients
A group of 154 patients with AML aged 60 years or older were included in the present study. All patients were enrolled between May 1997 and February 1999 in an international, multigroup, randomized phase 3 trial performed under auspices of the Dutch–Belgian Hemato-Oncology Cooperative Group and the UK Medical Research Council [14]. In that trial, 419 eligible white patients ≥60 years with previously untreated de novo and secondary AML (M0–M2 and M4–M7 according to the French–American–British [FAB] classification [15]) were randomized to receive two cycles of induction chemotherapy consisting of daunorubicin (DNR) and cytarabine (ara-C) with or without the P-gp inhibitor PSC-833 (Valspodar, Amdray®; Novartis Pharma, Basle, Switzerland). Patients in both arms in complete remission after these two cycles were to receive one consolidation consisting of ara-C, MXT, and etoposide. Inclusion criteria, clinical characteristics, treatment, and outcome of the phase 3 trial have been previously reported [14].
Bone marrow (BM) aspirates had been collected at diagnosis for the analysis of P-gp function and expression, as described previously [14]. Selection of patients for our study was based on availability of sufficient purified AML blast samples in our tissue bank, which was the case for 154 patients.
This study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before randomization.
Methods
BM aspirates were obtained in heparinized tubes. Mononuclear BM cells were collected by Ficoll Hypaque density gradient centrifugation (density 1.077 g/m3; Pharmacia, Uppsala, Sweden). To obtain purified samples with more than 85% of blasts, T-cell depletion and adherence depletion was performed as previously described [16]. Cells were cryopreserved in Dulbecco modified Eagle medium (DMEM; Gibco, Paisley, UK) supplemented with 10% dimethyl sulfoxide (Merck, Darmstadt, Germany) and 20% fetal calf serum (FCS; Gibco) and stored in liquid nitrogen. On the day of the experiments, BM cells were thawed. Cells were washed and resuspended in DMEM supplemented with 10% FCS. Before RNA and DNA isolation, cells were washed with phosphate-buffered saline (Gibco).
MDR1, MRP1, LRP, and BCRP mRNA analysis
The drug resistance proteins were analyzed using the methods that we previously reported [11]. In brief, total RNA was isolated using the TRISOLV™ extraction as described by the manufacturer (Biotecx, Houston, TX). RNA was aliquoted and stored at −80°C. RNA samples were analyzed for RNA integrity by gel electrophoresis. cDNA was synthesized by the use of the TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA), diluted, aliquoted, and stored at −80°C. Quantitative RT-PCR was used to measure the mRNA expression levels of MDR1, MRP1, LRP, and BCRP by Taqman-chemistry on an ABI PRISM 7700 sequence detector (Applied Biosystems) using two endogenous reference genes, i.e., glyceraldehyde-3-phosphate dehydrogenase and porphobilinogen deaminase.
Definition of endpoints
The clinical endpoints have been defined previously [14]. In brief, complete response (CR) was defined as a normocellular BM with <5% blasts, no Auer rods, and no evidence of extramedullary involvement. Because data on peripheral blood recovery within 60 days were not always available, they were not considered as a criterium for CR. Patients who relapsed or died within 28 days after CR were considered as not having achieved a CR. Event-free survival (EFS) was calculated from the date of randomization until no CR on induction therapy, relapse after CR, or death in CR, whichever came first. Patients who did not reach CR were considered failure for EFS at 1 day after randomization. Disease-free survival (DFS) was determined for all patients who achieved CR on induction therapy and was calculated from the date of CR until relapse or death, whichever came first. Overall survival (OS) was measured from randomization until death from any cause. Patients who were still alive at the date of last contact were then censored.
Statistical analysis
The original phase 3 trial had been designed to detect with a power of 80% an increase in 2-year EFS from 9.5% in the control arm (without PSC-833) to 18% in the PSC-833 arm (two-sided significance level α = 0.05) and included 419 eligible patients.
mRNA data were obtained from a subset of 154 patients with sufficient BM samples in our tissue bank available for analysis. Baseline parameters of interest were MDR1, MRP1, LRP, and BCRP mRNA expression. Clinical endpoints were CR rate, EFS, DFS, and OS.
Baseline characteristics of patients with or without mRNA expression data available were compared using the Fisher exact test or the Pearson χ 2 test in case of discrete variables, whichever appropriate, or the Wilcoxon rank-sum test in case of continuous variables. The association between patient baseline characteristics and mRNA expression levels was analyzed using the Pearson χ 2 test of the Spearman rank correlation test, whichever was appropriate. The prognostic value of mRNA levels with respect to CR rate was determined using logistic regression [17] whereas the impact of MDR1, MRP1, LRP, and BCRP on EFS, DFS, and OS was analyzed with Cox regression analysis [18]. For this purpose, the natural logarithm of the mRNA expression levels of the four resistance genes were included in the analyses because of the very skewed distribution of the original mRNA levels. In addition, the outcome of patients with coexpression of MDR1 and BCRP was evaluated to confirm the poor prognosis of AML with MDR1/BCRP coexpression reported by Benderra et al. [19] in patients with a median age of 45 years or older. These patients were defined as having mRNA levels of these two drug resistance genes equal to or higher than the median. Their outcome was compared to the other patients with at least one of the MDR1 and BCRP mRNA expression levels below the median. Logistic regression and Cox regression analyses were performed unadjusted, as well as adjusted for other prognostic factors, i.e., secondary AML, natural logarithm of WBC count, square root of percentage CD34+ cells, and cytogenetic risk (favorable/intermediate versus unfavorable versus unknown), as well as for treatment arm in the phase 3 trial, as about half of the patients had been randomized to receive PSC-833 in addition to their chemotherapy. Kaplan–Meier curves [20] were generated to illustrate survival and were compared using the log-rank test [21]. All reported p values are two-sided and, in view of the exploratory nature of these analyses, were calculated without adjustment for multiple testing. p values ≤ 0.05 were considered statistically significant.
Results
In the phase 3 trial, a total of 419 untreated patients with AML aged 60 years and older were randomized to receive two induction cycles with or without PSC-833. As reported, no difference was found between both treatment arms as regards CR rate (54% in the PSC-833 arm versus 48% in the control arm, p = 0.22), 5-year EFS (7 versus 8%, p = 0.53) nor DFS (13 versus 17%, p = 0.06) and OS (10% in both arms, p = 0.52) [14]. We previously reported the role of functional MDR1 expression with respect to clinical outcome in these patients.
In 154/419 of the patients, sufficient BM cells were available in our tissue bank to investigate the mRNA expression level of the drug resistance genes MDR1, MRP1, LRP, and BCRP. This subgroup consisted of a representative group according to age, gender, CD34 expression, cytogenetics, and FAB classification (Table 1). In this test group, a higher WBC count at diagnosis was observed than in the other 265 patients, and relatively more patients had been randomized to the PSC-833 arm (57 versus 45%, p = 0.02). There was no significant difference in the levels of MDR1, MRP1, LRP, nor BCRP mRNA expression between the two treatment arms (data not shown). The CR rate and survival endpoints were also similar in both patient groups (Table 1). However, patients with mRNA data in the PSC-833 arm had a higher CR rate (61 versus 40%, p = 0.02), whereas this was 54 versus 48% (p = 0.22) in all 419 patients.
The mRNA expression levels of the resistance genes were not significantly associated with the age of the patients (Table 2). MRP1 and LRP expression showed a strong positive correlation with WBC count. Negative associations of MDR1 and BCRP with WBC count were observed. A significant positive association was found between CD34 and MDR1 and also with BCRP mRNA expression. No significant correlation was found between MRP1 nor LRP, and CD34 expression (Table 2). Interestingly, secondary AML cases had a significantly higher expression of BCRP (p < 0.05) and lower MRP1 and LRP levels (both p < 0.01, Table 2). In the vast majority of our patients, also P-gp efflux and expression data were available. Function and expression data and MDR1 mRNA expression levels were highly correlated (p < 0.001), which was published recently [22].
In this cohort of patients of higher age with AML, MDR1 and BCRP were highly associated (p < 0.001), just as were MRP1 and LRP mRNA (p < 0.001; Fig. 1). A negative association was found between BCRP and MRP1 and between BCRP and LRP (both p < 0.001; Fig. 1). The 40 patients with coexpression of BCRP and MDR1 had significantly higher CD34 expression (median 39.5% [range 0.1–97.7%] versus 25.9% [range 0.1–97.9%]; p = 0.001) and a lower WBC count (median 4.5 [range 0.8–300]×109/l versus 28.1 [range 0.1–389]×109/l; p < 0.001). No significant correlation of MDR1, BCRP, or coexpression of MDR1 and BCRP was found with unfavorable cytogenetics (p = 0.4; Table 2).
Association between MDR1, MRP1, LRP, and BCRP mRNA expression levels. Each dot represents the expression of two drug resistance genes in one patient. The Spearman rank correlation coefficient has been calculated, along with the corresponding p value. Both the x- and y-axis have a logarithmic scale trim(X)* indicates that the 2.5% smallest and largest values of X have been shrinked; r, Spearman rank correlation coefficient; and p, p value. The results show a significant positive correlation between MDR1 and BCRP mRNA expression as illustrated by the p value and correlation coefficient. In addition, MRP1 and LRP are highly associated. BCRP shows a negative correlation with MRP1 and LRP
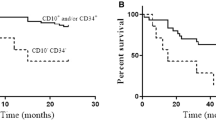
To assess the clinical relevance of the four resistance genes, their expression was evaluated with regard to CR rate and survival data, respectively. The median follow-up of the 25 patients still alive was 58 months (range, 1–80 months). Univariate logistic regression analysis showed that higher MDR1 mRNA expression predicted for a lower CR rate (log[MDR1]: odds ratio [OR]=0.75, 95% confidence interval [CI] 0.61–0.93, p = 0.009), whereas MRP1, LRP, and BCRP mRNA were not associated with CR (Fig. 1). MDR1 expression was also associated with a worse EFS (log[MDR1]: hazard ratio [HR]=1.14, 95% CI 1.03–1.27, p = 0.01) and OS (log[MDR1]: HR = 1.16, 95% CI 1.05–1.29, p = 0.004). Similar results were also obtained for MDR1/BCRP coexpression (Table 3; Fig. 2). When the analyses were performed with adjustment for other prognostic factors, as described in the “Statistical analysis”, only MDR1/BCRP mRNA coexpression remained significantly associated with a lower CR rate (OR = 0.37, 95% CI 0.15–0.91, p = 0.03), whereas a trend was observed for worse EFS (Table 3). On the other hand, higher CD34 expression was significantly associated with a lower CR rate (square root[CD34]: OR = 0.86, 95% CI 0.76–0.98, p = 0.02) and with worse EFS (HR = 1.12, 95% CI 1.06–1.19, p < 0.001), DFS (HR = 1.19, 95% CI 1.09–1.30, p < 0.001), and OS (HR = 1.17, 95% CI = 1.10–1.25, p < 0.001).
Discussion
This is the first comprehensive analysis of the effect of the major classical MDR genes in a cohort of elderly patients with AML homogeneously treated in a prospective clinical trial [14]. A wide range of expression of the various resistance genes was observed, consistent with previous studies and with comparable median values [9–11, 23]. Our results show that MRP1, LRP, and BCRP are not associated with CR rate or survival endpoints in patients with AML aged 60 years or older, indicating that the clinical relevance of the expression of these genes is limited in this patient population. This study confirms previous reports, which showed the unique prognostic role of MDR1 expression—which was however highly correlated with CD34 expression—in drug resistance in elderly AML (Table 3), in contrast to the prognostic value of MRP1 expression in AML, which has shown conflicting results, whereas currently, LRP is no longer thought to be important for clinical drug resistance [4, 5, 7, 24–27]. Recently, two studies in, respectively, 40 and 31 adult AML patients showed no effect of BCRP gene expression on CR rate, whereas OS was lower in patients with the highest BCRP expression [10, 23]. Damiani et al. [28] showed that BCRP expression did not influence achievement of complete remission in AML patients with a median age of 53 years and normal karyotype, however, BCRP expression was associated with higher relapse rate. In 59 children with de novo AML, a higher BCRP expression was observed in patients who did not reach CR, but this was not translated in poorer survival [29]. Benderra et al. [19] indicated that BCRP gene expression was an adverse prognostic factor for CR in a group of 149 relatively younger adult AML patients but only in patients treated with DNR and MXT and not with idarubicin. In our cohort of elderly AML patients who were all treated with DNR, whereas MXT was given as consolidation therapy after reaching CR, a significant correlation of BCRP mRNA expression with lower CR rate could not be shown.
Our study confirms that BCRP and MDR1 are coexpressed in AML patients with higher age as has been suggested previously from studies in smaller groups of relatively younger AML patients [9–11, 28]. Until now, only two studies have evaluated the clinical value of coexpression of MDR1 and BCRP in a sufficient number, although relatively younger adult AML patients [19, 28]. Benderra et al. [19] showed that CR rate was only 45% in the patients with coexpression of BCRP and MDR1 (+/+) in comparison with 66% in the MDR1/BCRP−/+ and +/−group and 90% in the MDR1/BCRP−/−group (p = 0.003). Moreover, a significantly lower DFS and OS were found in the MDR1/BCRP+/+group. Damiani et al. [28] found a trend towards a higher relapse rate in the small group of BCRP+/MDR1+patients, indicating that this represents a robust resistant AML phenotype, consistent with our findings in elderly AML. The recent finding that BCRP and MDR1 expression was mainly found in the most resistant group of AML, using gene expression profiling, underscores the role of these drug resistance genes in AML [30].
However, this study shows, that the prominent prognostic role of CD34 expression in elderly AML should be emphasized, as higher CD34 expression was adversely associated with all clinical endpoints. MDR1 and BCRP but not MRP1 and LRP mRNA expression were found to be associated with high CD34 expression in these elderly AML patients, which may explain why MDR1 was no longer significant for CR rate, EFS, and OS when adjusted for other prognostic variables including CD34. In the past, MDR1 expression has been linked to the CD34-positive hematopoietic stem cell compartment of the leukemia subtype. In two other studies in younger AML patients, no overexpression (on mRNA and protein level) of BCRP in the CD34-positive blast population of clinical AML samples was found [13, 19]. In contrast, earlier studies in mice demonstrated high levels of BCRP and MDR1 expression in normal hematopoietic stem cells [31–34]. Previously, BCRP expression in subsets of stem cells has been reported, indicating that high BCRP expression may exist in CD34+/CD38− cells or in CD34+/CD33−cells [12, 35]. The differential expression of BCRP and MDR1 in specific subsets of hematopoietic stem cells is consistent with the side population phenotype as proposed by Goodell et al. [36] who claim that BCRP expression can be separated from those expressing the other ABC proteins. This would suggest that BCRP is expressed in even less differentiated hematopoietic stem cells than MDR1 [19]. In our study in AML, these immature subsets could not be separately investigated, however, the unique BCRP/MDR1+/+subgroup of patients reflects an immature leukemic cell type that has a very resistant phenotype in vivo, illustrated by a low CR rate and poor outcome (Table 3; Fig. 2).
This is the first study in which a correlation was found between secondary AML and a high expression of BCRP mRNA but not the other resistance proteins. In addition to our previous report that BCRP is frequently upregulated in patients with AML at relapse, we now demonstrate that expression of BCRP is representative of secondary AML, which is especially observed in elderly patients [11, 29]. Recently, Ross [37] suggested that MDR modifiers may be of benefit for patients with multiple dysplastic features. This may suggest that BCRP is upregulated in diseases in which exposure to xenobiotics during life plays an etiologic role.
We conclude that coexpression of CD34-related coexpression of MDR1 and BCRP reflects a clinically resistant subgroup of elderly AML. In this age group, only BCRP is correlated with secondary AML. As such, the development of new treatment strategies for elderly AML patients may focus on modulation of drug resistance targeting both BCRP and MDR1.
References
Hunault M, Zhou D, Delmer A, Ramond S, Viguie F, Cadiou M et al (1997) Multidrug resistance gene expression in acute myeloid leukemia: major prognosis significance for in vivo drug resistance to induction treatment. Ann Hematol 74(2):65–71
Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS et al (1999) Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood 94(3):1086–1099
van den Heuvel-Eibrink MM, van der Holt B, te Boekhorst PA, Pieters R, Schoester M, Lowenberg B et al (1997) MDR 1 expression is an independent prognostic factor for response and survival in de novo acute myeloid leukaemia. Br J Haematol 99(1):76–83
Willman CL (1997) The prognostic significance of the expression and function of multidrug resistance transporter proteins in acute myeloid leukemia: studies of the Southwest Oncology Group Leukemia Research Program. Semin Hematol 34(4 Suppl 5):25–33
Mossink MH, van Zon A, Scheper RJ, Sonneveld P, Wiemer EA (2003) Vaults: a ribonucleoprotein particle involved in drug resistance? Oncogene 22(47):7458–7467
van der Kolk DM, de Vries EG, Noordhoek L, van den Berg E, van der Pol MA, Muller M et al (2001) Activity and expression of the multidrug resistance proteins P-glycoprotein, MRP1, MRP2, MRP3 and MRP5 in de novo and relapsed acute myeloid leukemia. Leukemia 15(10):1544–1553
van Zon A, Mossink MH, Schoester M, Scheper RJ, Sonneveld P, Wiemer EA (2004) Efflux kinetics and intracellular distribution of daunorubicin are not affected by major vault protein/lung resistance-related protein (vault) expression. Cancer Res 64(14):4887–4892
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK et al (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95(26):15665–15670
Ross DD, Karp JE, Chen TT, Doyle LA (2000) Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood 96(1):365–368
Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A et al (2005) BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res 29(2):141–146
van den Heuvel-Eibrink MM, Wiemer EA, Prins A, Meijerink JP, Vossebeld PJ, van der Holt B et al (2002) Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 16(5):833–839
van der Kolk DM, Vellenga E, Scheffer GL, Muller M, Bates SE, Scheper RJ et al (2002) Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood 99(10):3763–3770
van der Pol MA, Broxterman HJ, Pater JM, Feller N, van der Maas M, Weijers GW et al (2003) Function of the ABC transporters, P-glycoprotein, multidrug resistance protein and breast cancer resistance protein, in minimal residual disease in acute myeloid leukemia. Haematologica 88(2):134–147
van der Holt B, Lowenberg B, Burnett AK, Knauf WU, Shepherd J, Piccaluga PP et al (2005) The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood 106(8):2646–2654
Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD et al (1990) Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol 8(5):813–819
Lowenberg B, van Putten WL, Touw IP, Delwel R, Santini V (1993) Autonomous proliferation of leukemic cells in vitro as a determinant of prognosis in adult acute myeloid leukemia. N Engl J Med 328(9):614–619
Hosmer DW, Lemeshow S (1989) Applied logistic regression. Wiley, New York, NY
Cox DR (1972) Regression models and life tables. J R Stat Soc 34:187–220
Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP et al (2004) Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res 10(23):7896–7902
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50(3):163–170
van der Holt B, Van den Heuvel-Eibrink MM, Van Schaik RH, van der Heiden IP, Wiemer EA, Vossebeld PJ et al (2006) ABCB1 gene polymorphisms are not associated with treatment outcome in elderly acute myeloid leukemia patients. Clin Pharmacol Ther 80(5):427–439
Suvannasankha A, Minderman H, O’Loughlin KL, Nakanishi T, Ford LA, Greco WR et al (2004) Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br J Haematol 127(4):392–398
Legrand O, Simonin G, Zittoun R, Marie JP (1998) Lung resistance protein (LRP) gene expression in adult acute myeloid leukemia: a critical evaluation by three techniques. Leukemia 12(9):1367–1374
Baldus C, Fietz T, Rieder H, Schwartz S, Thiel E, Knauf W (2001) MDR-1 expression and deletions of chromosomes 7 and 5(Q) separately indicate adverse prognosis in AML. Leuk Lymphoma 40(5–6):613–623
Filipits M, Pohl G, Stranzl T, Suchomel RW, Scheper RJ, Jager U et al (1998) Expression of the lung resistance protein predicts poor outcome in de novo acute myeloid leukemia. Blood 91(5):1508–1513
Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM et al (1997) Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood 89(9):3323–3329
Damiani D, Tiribelli M, Calistri E, Geromin A, Chiarvesio A, Michelutti A et al (2006) The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica 91(6):825–828
Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A (2002) BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia 16(8):1443–1447
Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R et al (2006) Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood 108(2):685–696
Abbott BL, Colapietro AM, Barnes Y, Marini F, Andreeff M, Sorrentino BP (2002) Low levels of ABCG2 expression in adult AML blast samples. Blood 100(13):4594–4601
Abbott BL (2003) ABCG2 (BCRP) expression in normal and malignant hematopoietic cells. Hematol Oncol 21(3):115–130
Israeli D, Ziaei S, Gonin P, Garcia L (2005) A proposal for the physiological significance of mdr1 and Bcrp1/Abcg2 gene expression in normal tissue regeneration and after cancer therapy. J Theor Biol 232(1):41–45
Zhou S, Zong Y, Lu T, Sorrentino BP (2003) Hematopoietic cells from mice that are deficient in both Bcrp1/Abcg2 and Mdr1a/1b develop normally but are sensitized to mitoxantrone. BioTechniques 35(6):1248–1252
Raaijmakers MH, de Grouw EP, Heuver LH, van der Reijden BA, Jansen JH, Scheper RJ et al (2005) Breast cancer resistance protein in drug resistance of primitive CD34+38-cells in acute myeloid leukemia. Clin Cancer Res 11(6):2436–2444
Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G et al (1997) Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med 3(12):1337–1345
Ross DD (2004) Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome. Best Pract Res Clin Haematol 17(4):641–651
Acknowledgements
We acknowledge M. Schoester and A. Prins for their technical assistance and Dr. P.P. Piccaluga and Dr. P.J.M. Vossebeld for their contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van den Heuvel-Eibrink, M.M., van der Holt, B., Burnett, A.K. et al. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol 86, 329–337 (2007). https://doi.org/10.1007/s00277-007-0269-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-007-0269-7