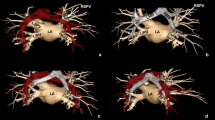
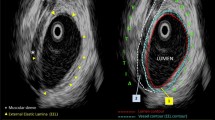
Abstract
Purpose
To determine the size of pulmonary artery (PA) at risk for occlusion during percutaneous microwave ablation and to assess the effect of vessel diameter, number, and patency, on ablation zone volume.
Materials and Methods
Computed tomography (CT) fluoroscopy-guided percutaneous microwave ablations were performed in 8 pigs under general anesthesia. All ablations were performed at 65 W for 5 min with a single 17-gauge antenna positioned in the central third of the lungs. A CT pulmonary angiogram was performed immediately after the ablations. The maximum diameter, number and patency of PA branches within each ablation zone were recorded. Ablation volumes were measured at gross dissection and with CT. Student’s t test was used to compare ablation zone volumes among groups.
Results
Twenty-one pulmonary ablations were performed. Six of the ablation zones (29%) contained at least 1 occluded PA branch. The mean diameter of the occluded PA branches in the ablation zones (2.4 mm; range, 2.0–2.8 mm) was significantly smaller than non-occluded PA branches (3.7 mm; range: 2.1–6.9 mm; p = 0.009). No PA branches ≥3 mm in size were occluded. There was no significant difference in volume of gross ablation zones that contained occluded versus non-occluded PAs (p = 0.42), one versus multiple PAs (p = 0.71), or PAs <3 mm versus ≥3 mm in diameter (p = 0.44).
Conclusions
PAs ≥3 mm in size have a low risk for iatrogenic occlusion during percutaneous microwave ablation. The presence of multiple adjacent PA branches, an occluded PA branch, and a vessel diameter ≥3 mm within the ablation zone had no observed effect on ablation zone volume.





Similar content being viewed by others
References
Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621–8.
Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non–small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:160–6.
Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–9.
de Baère T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. Am J Roentgenol. 2003;181:695–700.
Sakurai J, Mimura H, Gobara H, Hiraki T, Kanazawa S. Pulmonary artery pseudoaneurysm related to radiofrequency ablation of lung tumor. Cardiovasc Intervent Radiol. 2010;33:413–6.
Yamakado K, et al. Massive hemoptysis from pulmonary artery pseudoaneurysm caused by lung radiofrequency ablation successful treatment by coil embolization. Cardiovasc Intervent Radiol. 2010;33:410–2.
Anai H, et al. Effects of blood flow and/or ventilation restriction on radiofrequency coagulation size in the lung: an experimental study in swine. Cardiovasc Intervent Radiol. 2006;29(5):838–45.
Hiraki T, et al. Radiofrequency ablation of normal lungs after pulmonary artery embolization with use of degradable starch microspheres: results in a porcine model. J Vasc Interv Radiol. 2006;17(2):1991–8.
Oshima F, et al. Lung radiofrequency ablation with and without bronchial occlusion: experimental study in porcine lungs. J Vasc Interv Radiol. 2004;15(12):1451–6.
Iguchi T, Hiraki T, Gobara H, Mimura H, Fujiwara H, Tajiri N, et al. Percutaneous radiofrequency ablation of lung tumors close to the heart or aorta: evaluation of safety and effectiveness. J Vasc Interv Radiol. 2007;18(6):733–40.
Hiraki T, et al. Repeat radiofrequency ablation for local progression of lung tumors: does it have a role in local tumor control? J Vasc Interv Radiol. 2008;19(5):706–11.
Steinke K, et al. Effect of vessel diameter on the creation of ovine lung radiofrequency lesions in vivo: preliminary results. J Surg Res. 2005;124(1):85–91.
Yashiro H, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Interv Radiol. 2013;24(6):813–21.
Brace CL, et al. Pulmonary thermal ablation: Comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(3):705–11.
Andreano A, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6):2967–73.
Wright AS, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(1):132–9.
Nam CY, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19(7):1087–92.
Laeseke PF, et al. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20(9):1224–9.
Crocetti L, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33(4):818–27.
Care IoLARCo, Animals UoL, Resources NIoHDoR. Guide for the care and use of laboratory animals.: National Academies; 1985.
Yamamoto A, Nakamura K, Matsuoka T, et al. Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. Am J Roentgenol. 2005;185:1299–306.
Goldberg SN, Gazelle GS, Compton CC, McLoud TC. Radiofrequency tissue ablation in the rabbit lung: efficacy and complications. Acad Radiol. 1995;2:776–84.
Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(3):135–43.
Vogl TJ, et al. Factors influencing local tumor control in patients with neoplastic pulmonary nodules treated with microwave ablation: a risk-factor analysis. Am J Roentgenol. 2013;200(3):665–72.
Egashira Y, Singh S, Bandula S, Illing R. Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: a retrospective single-center experience. J Vasc Interv Radiol. 2016;27(4):474–9.
Chiang J, Cristescu M, Lee M, et al. Vascular occlusion during microwave ablation of hepatocellular carcinomas. Radiology. 2016;281(2):617–24.
Acknowledgements
This study was funded in part by National Institutes of Health Grant R01 CA149379 and by the RSNA Research and Education Foundation, Fellow Research Grant. The authors wish to acknowledge veterinary technician Lisa Sampson, BS for her commitment to ensuring ethical care during the animal experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
George A. Carberry, Elisabetta Nocerino, Mircea M. Cristescu and Amanda R. Smolock declare that they have no conflict of interests. Fred T. Lee Jr. and Christopher L. Brace are paid consultants for NeuWave Medical, Inc.
Rights and permissions
About this article
Cite this article
Carberry, G.A., Nocerino, E., Cristescu, M.M. et al. Microwave Ablation of the Lung in a Porcine Model: Vessel Diameter Predicts Pulmonary Artery Occlusion. Cardiovasc Intervent Radiol 40, 1609–1616 (2017). https://doi.org/10.1007/s00270-017-1689-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1689-y