Abstract
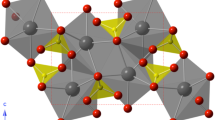
The Raman and Cr3+ and V2+ luminescence spectra of beryl and emerald have been characterized up to 15.0 and 16.4 GPa, respectively. The Raman spectra show that an E 1g symmetry mode at 138 cm−1 shifts negatively by −4.57 (±0.55) cm−1/GPa, and an extrapolation of the pressure dependence of this mode indicates that a soft-mode transition should occur near 12 GPa. Such a transition is in accord with prior theoretical results. Dramatic changes in Raman mode intensities and positions occur between 11.2 and 15.0 GPa. These changes are indicative of a phase transition that primarily involves tilting and mild distortion of the Si6O18 rings. New Raman modes are not observed in the high-pressure phase, which indicates that the local bonding environment is not altered dramatically across the transition (e.g., changes in coordination do not occur). Both sharp line and broadband luminescence are observed for both Cr3+ and V2+ in emerald under compression to 16.4 GPa. The R-lines of both Cr3+ and V2+ shift to lower energy (longer wavelength) under compression. Both R-lines of Cr3+ split at ~13.7 GPa, and the V2+ R1 slope changes at this pressure and shifts more rapidly up to ~16.4 GPa. The Cr3+ R-line splitting and FWHM show more complex behavior, but also shift in behavior at ~13.7 GPa. These changes in the pressure dependency of the Cr3+ and V2+ R-lines and the changes in R-line splitting and FWHM at ~13.7 GPa further demonstrate that a phase transition occurs at this pressure, in good agreement with our Raman results. The high-pressure phase of beryl appears to have two Al sites that become more regular under compression. Hysteresis is not observed in our Raman or luminescence spectra on decompression, suggesting that this transition is second order in nature: The occurrence of a second-order transition near this pressure is also in accord with prior theoretical results. We speculate that the high-pressure phase (beryl-II) might be a mildly modulated structure, and/or that extensive twinning occurs across this transition.











Similar content being viewed by others
References
Aurisicchio C, Fioravanti G, Grubessi O, Zanazzi PF (1988) Reappraisal of the crystal chemistry of beryl. Am Miner 73:826–837
Bray KL (2001) High pressure probes of electronic structure and luminescence properties of transition metal and lanthanide systems. In: Yersin H (ed) Transition metal and rare earth compounds. Springer, Berlin, Heidelberg, pp 1–94
Buchert J, Katz A, Alfano RR (1983) Laser action in emerald. IEE J Quantum Electron 19:1477–1478
Burns R (1993) Mineralogical applications of crystal field theory, 2nd edn. Cambridge University Press, Cambridge
Canny B, Chervin JD, Curie D, Gonzalez J, Berry D, Ho SA (1987) High pressure properties of some laser materials. In: Di Bartolo B (ed) Spectroscopy of solid-state laser-type materials. Springer, US, pp 431–449
Chopelas A, Nicol M (1982) Pressure dependence to 100 kilobars of the phonons of MgO at 90 and 295 K. J Geophys Res 87:8591–8597
Fan D, Xu J, Kuang Y, Li X, Li Y, Xie H (2015) Compressibility and equation of state of beryl (Be3Al2Si6O18) by using a diamond anvil cell and in situ synchrotron X-ray diffraction. Phys Chem Miner 42:529–539
Finkelstein GJ, Dera PK, Duffy TS (2015) High-pressure phases of cordierite from single-crystal X-ray diffraction to 15 GPa. Am Miner 100:1821–1833
Fridrichová J, Bačík P, Bizovská V, Libowitzky E, Škoda R, Uher P, Ozdin D, Števko M (2016) Spectroscopic and bond-topological investigation of interstitial volatiles in beryl from Slovakia. Phys Chem Miner 43:419–437
Fujishiro I, Piermarini GJ, Block S, Munro RG (1982) Viscosities and glass transition pressures in the methanol–ethanol–water system. In: High pressure research in science and industry: proceedings of the 8th AIRAPT conference, vol 2, pp 608–611
Gaft M, Reisfeld R, Panczer G (2005) Modern luminescence spectroscopy of minerals and materials. Springer, Berlin
Gaudry E, Cabaret D, Brouder C, Letard I, Rogalev A, Wilhlem F, Jaouen N, Sainctavit P (2007) Relaxations around the substitutional chromium site in emerald: X-ray absorption experiments and density functional calculations. Phys Rev B 76:094110
Gibbs G, Breck D, Meagher E (1968) Structural refinement of hydrous and anhydrous synthetic beryl and emerald. Lithos 1:275–285
Hagemann H, Lucken A, Bill H, Gysler-Sanz J, Stalder HA (1990) Polarized Raman spectra of beryl and bazzite. Phys Chem Miner 17:395–401
Hazen RM, Au AY, Finger W (1986) High-pressure crystal chemistry of beryl (Be3Al2Si6O18) and euclase (BeAlSiO4OH). Am Miner 71:977–984
Hemingway BS, Barton MD, Robie RA, Haselton HT (1986) Heat capacities and thermodynamic functions for beryl, Be3Al2Si6O18, phenakite, Be2SiO4, euclase, BeAlSiO4(OH), bertrandite Be4Si2O7(OH)2, and chrysoberyl BeAl2O4. Am Miner 71:557–568
Hochella MF, Brown GE (1986) Structural mechanisms of anomalous thermal expansion of cordierite-beryl and other framework silicates. J Am Ceram Soc 69:13–18
Hoemmerich U, Bray KL (1995) Direct observation of anticrossing behaviour in a luminescent Cr3+-doped system. Phys Rev B 51:8595–8598
Hofmeister AM, Hoering TC, Virgo D (1987) Vibrational spectroscopy of beryllium aluminosilicates: heat capacity calculations from band assignments. Phys Chem Miner 14:205–224
Imbusch GF, Yen WM, Schawlow AL, McCumber DE, Sturge M (1964) Temperature dependence of the width and position of the 2E 4A2 fluorescence lines of Cr3+ and V2+ in MgO. Phys Rev 133:A1029–A1034
Jovanić BR (1997) High pressure and fluorescence lifetime of the 2E level in MgO:Cr3+. Phys Scr 56:477–479
Jovanić BR (2000) Effect of high pressure on fluorescence lifetime and position for R1 line in synthetic spinel MgAl2O4:Cr3+. Mater Sci Forum 352:247–250
Jovanić BR, Viana B, Radenković B, Despotović M, Panić B (2010) High-pressure optical studies of LMA: V2+. Mater Chem Phys 124:109–112
Kisliuk P, Moore CA (1967) Radiation from the 4T2 State of Cr3+ in ruby and emerald. Phys Rev 160:307–312
Klotz S, Chervin J-C, Munsch P, Le Marchand G (2009) Hydrostatic limits of 11 pressure transmitting media. J Phys D Appl Phys 42:075413
Kottke T, Williams F (1983) Pressure dependence of the alexandrite emission spectrum. Phys Rev B 28:1923–1927
Koziarska B, Godlewski M, Suchocki A, Czaja M, Mazurak Z (1994) Optical properties of zoisite. Phys Rev B 50:297–300
Lai ST (1987) Highly efficient emerald laser. J Opt Soc Am B 4:1286–1290
Likhacheva AY, Goryainov SV, Krylov AS, Bul’bak TA, Prasad P (2012) Raman spectroscopy of natural cordierite at high water pressure up to 5 GPa. J Raman Spectrosc 43:559–563
Mao HK, Xu JA, Bell PM (1986) Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–4676
Mei Y, Peng RM, Chen BW, Zheng WC (2016) Local compressibilities of Cr3+ octahedral clusters in the natural and synthetic emerald crystals. Optik 127:5152–5154
Milberg ME, Blair HD (1977) Thermal expansion of cordierite. J Am Ceram Soc 60:372–373
Miletich R, Gatta GD, Willi T, Mirwald PW, Lotti P, Merlini M (2014a) Cordierite under hydrostatic compression: anomalous elastic behavior as a precursor for a pressure-induced phase transition. Am Mineral 99:479–493
Miletich R, Scheidl KS, Schmitt M, Moissl AP, Pippinger T, Gatta GD, Schuster B, Trautmann C (2014b) Static elasticity of cordierite I: effect of heavy ion irradiation on the compressibility of hydrous cordierite. Phys Chem Miner 41:579–591
Morosin B (1972) Structure and thermal expansion of beryl. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem 28:1899–1903
O’Bannon E, Williams Q (2016) A Cr3+ luminescence study of spodumene at high pressures: effects of site geometry, a phase transition and a level-crossing. Am Miner 101:1406–1413
Ohkura H, Hashimoto H, Mori Y, Chiba Y, Isotani S (1987) The luminescence and ESR of a synthetic emerald and the natural ones mined from Santa Terezinha in Brazil. Jpn J Appl Phys 26:1422–1428
Ollier N, Fuchs Y, Cavani O, Horn AH, Rossano S (2015) Influence of impurities on Cr3+ luminescence properties in Brazilian emerald and alexandrite. Eur J Miner 27:783–792
Parkin KM, Burns RG (1980) High temperature crystal field spectra of transition metal-bearing minerals: relevance to remote-sensed spectra of planetary surfaces. In: Lunar and planetary science conference proceedings, vol 11, pp 731–755
Piermarini GJ, Block S, Barnett JD (1973) Hydrostatic limits in liquids and solids to 100 kbar. J Appl Phys 44:5377–5382
Piermarini GJ, Block S, Barnett JD, Forman RA (1975) Calibration of the pressure dependence of the R1 ruby fluorescence line to 195 kbar. J Appl Phys 46:2774
Prencipe M, Nestola F (2005) Quantum-mechanical modeling of minerals at high pressures. The role of the Hamiltonian in a case study: the beryl (Al4Be6Si12O36). Phys Chem Miner 32:471–479
Prencipe M, Nestola F (2007) Minerals at high pressure. Mechanics of compression from quantum mechanical calculations in a case study: the beryl (Al4Be6Si12O36). Phys Chem Miner 34:37–52
Prencipe M, Noel Y, Civalleri B, Roetti C, Dovesi R (2006) Quantum-mechanical calculation of the vibrational spectrum of beryl (Al4Be6Si12O36) at the Γ point. Phys Chem Miner 33:519–532
Prencipe M, Scanavino I, Nestola F, Merlini M, Civalleri B, Bruno M, Dovesi R (2011) High-pressure thermo-elastic properties of beryl (Al4Be6Si12O36) from ab initio calculations, and observations about the source of thermal expansion. Phys Chem Miner 38:223–239
Putnis A, Bish DL (1983) The mechanism and kinetics of Al, Si ordering in Mg-cordierite. Am Miner 68:60–65
Qin S, Liu J, Li H, Zhu X, Li X (2008) In-situ high-pressure X-ray diffraction of natural beryl. Chin J High Press Phys 22:1–5
Quarles GJ, Suchocki A, Powell RC, Lai S (1988) Optical spectroscopy and four-wave mixing in emerald. Phys Rev B 38:9996–10006
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Sánchez-Alejo MA, Hernández-Alcántara JM, Jiménez C, Calderón T, García EC (2011) Optical spectroscopy and high pressure on emeralds: synthetic and natural. In: Proceedings of SPIE, the international society for optical engineering, vol 8011, pp 1–6
Sanz-Ortiz MN, Rodriguez F, Hernandez I, Valiente R, Kuck S (2010) Origin of the 2E–4T2 Fano-type resonance in Cr3+-doped LiCaAlF6: pressure-induced excited-state crossover. Phys Rev B 81:045114
Schlenker JL, Gibbs GV, Hill EG, Crews SS, Myers RH (1977) Thermal expansion coefficients for indialite, emerald, and beryl. Phys Chem Miner 1:243–255
Schmetzer K, Eysel HH (1974) Absorptions- and emissonsspektrum von V2+/V3+ in beryllen. Zeitschrift für Naturforsch A 29:1458–1460
Shen Y, Riedener T, Bray KL (2000) Effect of pressure and temperature on energy transfer between Cr3+ and Tm3+ in Y3Al5O12. Phys Rev B 61:11460–11471
Sherriff B, Grundy D, Hartman JS, Hawthorne F, Cerny P (1991) The incorporation of alkalis in beryl: multi-nuclear MAS NMR and crystal-structure study. Can Miner 29:271–285
Shinn MD, Tesar A (1992) Observation of temperature-dependent Cr3+–Nd3+ sensitization in fluoride glasses. J Lumin 51:189–195
Skvortsova V, Mironova-Ulmane N, Trinkler L, Merkulov V (2015) Optical properties of natural and synthetic beryl crystals. In: IOP conference series: materials science and engineering, vol 77, p 012034
Snytnikov VN, Stoyanovskii VO, Larina TV, Krivoruchko OP, Ushakov VA, Parmon VN (2008) Laser-induced luminescence of model Fe/Al2O3 and Cr/Al2O3 catalysts. Kinet Catal 49:291–298
Sugano S, Tanabe Y (1958) Absorption spectra of Cr3+ in Al2O3 part A. Theoretical studies of the absorption bands and lines. J Phys Soc Jpn 13:880–889
Syassen K (2008) Ruby under pressure. High Press Res 28:75–126
Taran MN, Langer K, Platonov AN, Indutny VV (1994) Optical absorption investigation of Cr3+ ion-bearing minerals in the temperature range 77–797 K. Phys Chem Miner 21:360–372
Taran MN, Ohashi H, Langer K, Vishnevskyy AA (2011) High-pressure electronic absorption spectroscopy of natural and synthetic Cr3+-bearing clinopyroxenes. Phys Chem Miner 38:345–356
Wamsley P, Bray K (1994) The effect of pressure on the luminescence of Cr3+:YAG. J Lumin 59:11–17
Williams Q, Jeanloz R (1985) Pressure shift of Cr3+-ion-pair emission lines in ruby. Phys Rev B 31:7449–7451
Wood DL, Nassau K (1967) Infrared spectra of foreign molecules in beryl. J Chem Phys 47:2220–2228
Xu J, Kuang Y, Zhang B, Liu Y, Fan D, Li X, Xie H (2016) Thermal equation of state of natural tourmaline at high pressure and temperature. Phys Chem Miner 43:315–326
Yamaga M, Henderson B (1991) Line shape and lifetimes of Cr3+ luminescence in silicate glasses. Phys Rev B 44:4853–4861
Yoon HS, Newnham RE (1973) The elastic properties of beryl. Acta Crystallogr Sect A 29:507–509
Zheng W-C (1995) Determination of the local compressibilities for Cr3+ ions in some garnet crystals from high-pressure spectroscopy. J Phys Condens Matter 7:8351–8356
Acknowledgments
Constructive and insightful comments from M. Prencipe and an anonymous reviewer, and helpful discussions with M. Merlini and C.M. Beavers, greatly improved the quality of this manuscript. E. O’Bannon thanks Juan Diego Prieto for the emerald sample. We thank Dan Sampson for invaluable technical assistance with the Raman spectrometer and Rob Franks for help with the trace element analysis. Work was partially supported by NSF through EAR-1215745, and COMPRES, the Consortium for Materials Properties Research in Earth Sciences under NSF Cooperative Agreement EAR 11-57758. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Bannon, E., Williams, Q. Beryl-II, a high-pressure phase of beryl: Raman and luminescence spectroscopy to 16.4 GPa. Phys Chem Minerals 43, 671–687 (2016). https://doi.org/10.1007/s00269-016-0837-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-016-0837-2