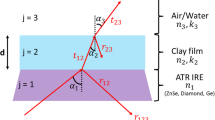
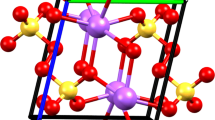
Abstract
Attenuated total reflectance (ATR) infrared spectra were measured on a synthetic and a natural fluorapatite sample. A modeling approach based on the computation of the Fresnel reflection coefficient between the ATR crystal and the powder sample was used to analyze the line shape of the spectra. The dielectric properties of the samples were related to those of pure fluorapatite using an effective medium approach, based on Maxwell–Garnett and Bruggeman models. The Bruggeman effective medium model leads to a very good agreement with the experimental data recorded on the synthetic fluorapatite sample. The poorer agreement observed on the natural sample suggests a more significant heterogeneity of the sample at a characteristic length scale larger than the mid-infrared characteristic wavelength, i.e., about 10 micrometers. The results demonstrate the prominent role of macroscopic electrostatic effects over fine details of the microscopic structure in determining the line shape of strong ATR bands.





Similar content being viewed by others
References
Adams DM, Gardner IR (1974) Single-crystal vibrational spectra of apatite, vanadinite and mimetite. J Chem Soc Dalton 14:1505–1509
Antonakos A, Liarokapis E, Leventouri T (2007) Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 28:3043–3054
Balan E, Mauri F, Lemaire C, Brouder C, Guyot F, Saitta AM, Devouard B (2002) Multiple ionic plasmon resonances in naturally-occurring multiwall nanotubes: infrared spectra of chrysotile asbestos. Phys Rev Lett 89:177401
Balan E, Delattre S, Guillaumet M, Salje EKH (2010) Low-temperature infrared spectroscopic study of OH stretching modes in kaolinite and dickite. Am Mineral 95:1257–1266
Balan E, Delattre S, Roche D, Segalen L, Morin G, Guillaumet M, Blanchard M, Lazzeri M, Brouder C, Salje EKH (2011) Line-broadening effects in the powder infrared spectrum of apatite. Phys Chem Miner 38:111–122
Beasley MM, Bartelink EJ, Taylor L, Miller RM (2014) Comparison of transmission FTIR, ATR, and DRIFT spectra: implications for assessment of bone bioapatite diagenesis. J Archaeol Sci 46:16–22
Bisal F, Hinman WC (1972) A method of estimating the apparent density of soil aggregates. Can J Soil Sci 52:513–514
Born M, Wolf E (1980) Principles of optics, 6th edn. Pergamon Press, New York
Bruggeman DAG (1935) Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann Phys 416:665–679
Cherkaeva E, Golden KM (1998) Inverse bounds for microstructural parameters of composite media derived from complex permittivity measurements. Wave Random Media 8(4):437–450
Chevrinais M, Balan E, Cloutier R (2016) New insights in the ontogeny and taphonomy of the devonian acanthodian Triazeugacanthus affinis from the Miguasha Fossil-Lagerstätte, Eastern Canada. Minerals 6:1
Elliott JC (2002) Calcium phosphate biominerals. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates: geochemical, geobiological, and materials importance, vol 48. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, Chantilly, Virginia, pp 427–453
Fleet ME (2009) Infrared spectra of carbonate apatites: ν2-region bands. Biomaterials 30:1473–1481
Fleet ME, Liu X (2008) Accommodation of the carbonate ion in fluorapatite synthesized at high pressure. Am Mineral 93:1460–1469
Gadenne M, Lafait J, Gadenne P (1989) Infrared absorption of Au-Al2O3 thin cermet films: experiment, Bruggeman model, far and near the percolation threshold. Phys A 157:400–406
Garnett JCM (1904) Colours in metal glasses and metallic films. Philos Trans R Soc A 203:385–420
Kendrick J, Burnett AD (2016) PDielec: the calculation of infrared and terahertz absorption for powdered crystals. J Comput Chem 37:1491–1504
Klee WE (1970) The vibrational spectra of the phosphate ions in fluorapatite. Z Kristallogr 131:95–102
Knudsen AC, Gunter ME (2002) Sedimentary phosphates—an example: phosphoria formation, Southern Idaho, U.S.A. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates: geochemical, geobiological and materials importance. Rev Mineral Geochem 48:363–389
Landauer R (1978) Electrical conductivity in inhomogeneous media. AIP Conf Proc 40:2–45
Lang L, Kirsimäe K, Vahur S (2016) Diagenetic fate of bioapatite in linguliform brachiopods: multiple apatite phases in shells of Cambrian lingulate brachiopod Ungula ingrica (Eichwald). Lethaia 49:13–27
Lebon M, Zazzo A, Reiche I (2014) Screening in situ bone and teeth preservation by ATR-FTIR mapping. Palaeogeogr, Palaeoclimatol 416:110–119
Leroy G, Leroy N, Penel G, Rey C, Laforgue P, Bres E (2000) Polarized micro-Raman study of fluorapatite single crystals. Appl Spectrosc 54(10):1521–1527
Leventouri T, Chakoumakos BC, Moghaddam HY, Perdikatsis V (2000) Powder neutron diffraction studies of a carbonate fluorapatite. J Mater Res 15:511–517
Levy O, Stroud D (1997) Maxwell Garnett theory for mixtures of anisotropic inclusions: application to conducting polymers. Phys Rev B 56:8035–8046
Meyer H-W, Carpenter MA, Becerro AI, Seifert F (2002) Hard-mode infrared spectroscopy of perovskites across the CaTiO3–SrTiO3 solid solution. Am Mineral 87:1291–1296
Michel V, Ildefonse P, Morin G (1995) Chemical and structural changes in Cervus Elaphus tooth enamels during fossilization (Lazaret cave): a combined IR and XRD Rietveld analysis. Appl Geochem 10:145–159
Morin G, Allard T, Balan E, Ildefonse P, Calas G (2002) Native Cd+ in sedimentary fluorapatite. Eur J Mineral 14:1087–1094
Nounah A, Lacout JL (1993) Thermal behavior of cadmium containing apatites. J Solid State Chem 107:444–451
Pan Y, Fleet M (2002) Compositions of the apatite-group minerals: substitution mechanisms and controlling factors. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates: geochemical, geobiological and materials importance. Rev Miner Geochem 48:13–49
Pucéat E, Reynard B, Lécuyer C (2004) Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem Geol 205:83–97
Reynard B, Balter V (2014) Trace elements and their isotopes in bones and teeth: diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeogr, Palaeoclimatol 416:4–16
Roche D, Segalen L, Balan E, Delattre S (2010) Preservation assessment of Miocene–Pliocene tooth enamel from Tugen Hills (Kenyan Rift Valley) through FTIR, chemical and stable-isotope analyses. J Archaeol Sci 37:1690–1699
Salje EKH, Bismayer U (1997) Hard mode spectroscopy: the concept and applications. Phase Transit 63:1–75
Salje E, Güttler B (1984) Anderson transition and intermediate polaron formation in WO3−x Transport properties and optical absorption. Philos Mag B 50:607–620
Salje EKH, Yagil Y (1996) Hard mode spectroscopy for the investigation of structural and superconducting phase transitions. J Phys Chem Solids 57:1413–1424
Salje EKH, Carpenter MA, Malcherek T, Boffa Balaran T (2000) Autocorrelation analysis of infrared spectra from minerals. Eur J Mineral 12:503–519
Shemesh A (1990) Crystallinity and diagenesis of sedimentary apatites. Geochim Cosmochim Acta 54:2433–2438
Shusko MY (2009) Effective permittivity of mixtures of anisotropic particles. J Phys D Appl Phys 42(15):155410
Sihvola AH, Kong JA (1988) Effective permittivity of dielectric mixtures. IEEE T Geosci Remote 26:420–429
Spanier JE, Herman IP (2000) Use of hybrid phenomenological and statistical effective-medium theories of dielectric functions to model the infrared reflectance of porous SiC films. Phys Rev B 61:10437–10450
Stathopoulou ET, Psycharis V, Chryssikos GD, Gionis V, Theodorou G (2008) Bone diagenesis: new data from infrared spectroscopy and X-ray diffraction. Palaeogeogr, Palaeoclimatol 266:168–174
Surovell TA, Stiner MC (2001) Standardizing infra-red measures of bone mineral crystallinity: an experimental approach. J Archaeol Sci 28:633–642
Thompson TJU, Gauthier M, Islam M (2009) The application of a new method of fourier transform infrared spectroscopy to the analysis of burned bone. J Archaeol Sci 36:910–914
Trueman CN, Privat K, Field J (2008) Why do crystallinity values fail to predict the extent of diagenetic alteration of bone mineral? Palaeogeogr, Palaeoclimatol 266:160–167
Walker D, Scharnberg K (1990) Electromagnetic response of high-Tc superconductors. Phys Rev B 42:2211–2221
Waychunas GA, Zhang H (2008) Structure, chemistry and properties of mineral nanoparticles. Elements 4:381–387
Weiner S, Bar-Yosef O (1990) States of preservation of bones from prehistoric sites in the Near East: a survey. J Archaeol Sci 17:187–196
Yagil Y, Baudenbacher F, Zhang M, Birch JR, Kinder H, Salje EKH (1995) Optical properties of YBa2Cu3O7−d thin films. Phys Rev B 52:15582–15591
Yi H, Balan E, Gervais C, Segalen L, Fayon F, Roche D, Person A, Morin G, Guillaumet M, Blanchard M, Lazzeri M, Babonneau F (2013) A carbonate-fluoride defect model for carbonate-rich fluorapatite. Am Mineral 98:1066–1069
Yi H, Balan E, Gervais C, Segalen L, Roche D, Fayon F, Person A, Morin G, Babonneau F (2014) Probing atomic scale transformation of fossil enamel using FTIR and NMR spectroscopy: a case study from the Tugen Hills (Rift Gregory, Kenya). Acta Biomater 10:3952–3958
Zhang M, Wruck B, Graeme Barber A, Salje EKH, Carpenter MA (1996) Phonon spectroscopy on alkali-feldspars: phase transitions and solid solutions. Am Mineral 81:92–104
Acknowledgments
We thank G. Morin for providing us with the fluorapatite samples. Support by M. Guillaumet, I. Estève and the IMPMC spectroscopy and SEM–FIB facilities is acknowledged. We thank Prof E.K.H. Salje and an anonymous reviewer for thoughtful and constructive reviews of this manuscript. This work was supported by French state funds managed by the ANR within the Investissements d’Avenir program under reference ANR-11-IDEX-0004-02 and, more specifically, within the framework of the Cluster of Excellence MATISSE led by Sorbonne Universités. Support from the Convergence Program “Environnements & Société” of Sorbonne Universités is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Complex solutions of the uniaxial Bruggeman model
The Bruggeman relation is (Eq. 2):
The solutions of the equation are the roots of a cubic polynomial with complex coefficients:
with
The three roots are:
Among these three roots, only that with a positive imaginary part has to be selected.
Some mathematical properties of the Bruggeman model
The solutions of Eq. 5 are the roots of a quadratic polynomial equation with complex coefficients:
with \(x = \frac{{\varepsilon_{Br} }}{{\varepsilon_{h} }}\), \(b = - \frac{{(3f - 1)\frac{{\varepsilon_{S} }}{{\varepsilon_{h} }} + (2 - 3f)}}{4}\) and \(c = - \frac{{\varepsilon_{S} }}{2{\varepsilon_{h}}}\). ε h is the dielectric constant of the host, and ε S = ε ′ S + iε ″ S is the complex dielectric function of the substance.
The corresponding solutions are: \(x_{ \pm } = - b \pm \sqrt \Delta\) with Δ = b 2 − c. We will now show that the imaginary part of the solution x + is positive.
The imaginary part of \(\sqrt \varDelta\) is equal to \(\sqrt {\left( {\left| \Delta \right| - \Delta_{r} } \right)/2}\) where Δ = Δ r + iΔ i . The imaginary part of x + is thus:
where b = b r + ib i
We now show that the absolute value |b i | is smaller than \(\sqrt {\frac{{\left( {\left| \Delta \right| - \Delta_{r} } \right)}}{2}}\). This is equivalent to determining that |Δ|2 − (2b 2 i + Δ r )2 is positive. Replacing the coefficients Δ and bi by their expressions as a function of the dielectric function of the substance and matrix, we obtain:
which is indeed always positive for 0 ≤ f ≤ 1.
As a consequence, \(\text{Im} (x_{ + } )\) is also positive. This property is even stronger than the Herglotz property because ε ″ S is not assumed to be positive. In particular for f = 0, Im(x+) = (ε ″ S + |ε ″ S |)/2 ≥ 0 even if ε ″ S is negative. The condition that ɛ h is real and positive thus implies that Im(ε Br ) is positive, for any function ε S .
As \(- b_{i} = (3f - 1)\frac{{\varepsilon^{\prime\prime}_{S} }}{{4\varepsilon_{h} }}\) and ε ″ S > 0, the first term in Eq. 6 is positive when f > 1/3. This contribution is thus proportional to the dielectric function of the pure bulk substance and always present. For f < 1/3, this contribution becomes negative, but since Im(x +) ≥ 0, this negative contribution is compensated for by the second term in Eq. 6. The ε ″ S peak then disappears completely from the spectrum. Equation 6 has another interesting consequence. In a region where |Δi| is smaller than |Δr|, there are two regimes: If Δr ≥ 0, then |Δ| − Δr ≈ Δ 2i /(2Δr) is small and the first term of Eq. 6 is dominant for f > 0.3; if Δr ≤ 0, then |Δ| − Δr ≈ 2|Δr| and the deformation of the spectrum due to the second term of Eq. 6 becomes large. In other words, the deformation of the spectrum of apatite for f > 0.3 occurs when Δ has a large negative real part.
We now examine the properties of the imaginary part of the solution for f = 0.5 and ε h = 1, i.e., the parameters used to model the spectrum of synthetic fluorapatite (Fig. 6). In this case, b 2 − c = ɛ S (ɛ S + 34) + 1. For a vanishingly small damping parameter and out of the TO frequency, ε S is real: ε S ≈ ε ′ S . Therefore, only the second term contributes to the imaginary part of the solution when b 2 − c is negative. This condition is met for ε ′ S approximately ranging between −34 and zero, i.e., on the high energy side of the TO frequency and up to about the LO frequency. For ε ′ S below −34, the imaginary part of ε S becomes dominant and the spectrum is proportional to the dielectric function of the pure bulk substance.
Real part of the model dielectric function (bold line, right axis) and imaginary part of the corresponding effective dielectric function (dotted line, left axis). The small damping parameter (1 cm−1) makes it possible to discriminate the two superposed contributions for a volume fraction of 0.5. The thin contribution at TO frequency displays an almost Lorentzian shape proportional to the imaginary part of the model dielectric function. In contrast, the broad contributions at higher frequency are observed when the real part of the model dielectric function takes values between ~−34 and ~0 (LO frequency), indicated by the horizontal dotted lines
Rights and permissions
About this article
Cite this article
Aufort, J., Ségalen, L., Gervais, C. et al. Modeling the attenuated total reflectance infrared (ATR-FTIR) spectrum of apatite. Phys Chem Minerals 43, 615–626 (2016). https://doi.org/10.1007/s00269-016-0821-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-016-0821-x