Abstract
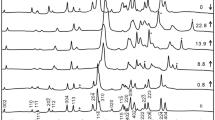
The stability of the high-pressure CaCO3 calcite (cc)-related polymorphs was studied in experiments that were performed in conventional diamond anvil cells (DAC) at room temperature as a function of pressure up to 30 GPa as well as in internally heated diamond anvil cells (DAC-HT) at pressures and temperatures up to 20 GPa and 800 K. To probe structural changes, we used Raman and FTIR spectroscopy. For the latter, we applied conventional and synchrotron mid-infrared as well as synchrotron far-infrared radiation. Within the cc-III stability field (2.2–15 GPa at room temperature, e.g., Catalli and Williams in Phys Chem Miner 32(5–6):412–417, 2005), we observed in the Raman spectra consistently three different spectral patterns: Two patterns at pressures below and above 3.3 GPa were already described in Pippinger et al. (Phys Chem Miner 42(1):29–43, 2015) and assigned to the phase transition of cc-IIIb to cc-III at 3.3 GPa. In addition, we observed a clear change between 5 and 6 GPa that is independent of the starting material and the pressure path and time path of the experiments. This apparent change in the spectral pattern is only visible in the low-frequency range of the Raman spectra—not in the infrared spectra. Complementary electronic structure calculations confirm the existence of three distinct stability regions of cc-III-type phases at pressures up to about 15 GPa. By combining experimental and simulation data, we interpret the transition at 5–6 GPa as a re-appearance of the cc-IIIb phase. In all types of experiments, we confirmed the transition from cc-IIIb to cc-VI at about 15 GPa at room temperature. We found that temperature stabilizes cc-VI to lower pressure. The reaction cc-IIIb to cc-VI has a negative slope of −7.0 × 10−3 GPa K−1. Finally, we discuss the possibility of the dense cc-VI phase being more stable than aragonite at certain pressure and temperature conditions relevant to the Earth’s mantle.












Similar content being viewed by others
References
Adams DM, Willimas AD (1980) Vibrational spectroscopy at very high pressures. Part 26. An infrared study of the metastable phases of CaCO3. J Chem Soc Dalton 8:1482–1486
Baroni S, de Gironcoli S, Dal Corso A, Giannozzi P (2001) Phonons and related crystal properties from density-functional perturbation theory. Rev Mod Phys 73:515–562
Boulard E, Gloter A, Corgne A, Antonangeli D, Auzende AL, Perrillat JP, Guyot F, Fiquet G (2011) New host for carbon in the deep Earth. PNAS 108:5184–5187
Boulard E, Pan D, Galli G, Liu Z, Mao WL (2015) Tetrahedrally coordinated carbonates in Earth’s lower mantle. Nat Commun 6:6311
Brenker FE, Vollmer C, Vincze L, Vekemans B, Szymanski A, Janssens K, Szaloki I, Nasdala L, Joswig W, Kaminsky F (2007) Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet Sci Lett 260:1–9
Bridgman PW (1939) The high pressure behavior of miscellaneous minerals. Am J Sci 237:7–18
Caracas R, Bobocioiu E (2011) The WURM project - a freely available web-based repository of computed physical data for minerals. Am Miner 96:437–443
Carteret C, De la Pierre M, Dossot M, Pascale F, Erba A, Dovesi R (2013) The vibrational spectrum of CaCO3 aragonite: a combined experimental and quantum-mechanical investigation. J Chem Phys 138:014201
Catalli K, Williams Q (2005) A high-pressure phase transition of calcite-III. Am Mineral 90(10):1679–1682
Catalli K, Santillán J, Williams Q (2005) A high pressure infrared spectroscopic study of PbCO3-cerussite: constraints on the structure of the post-aragonite phase. Phys Chem Miner 32(5–6):412–417
Cerantola V, McCammon C, Kupenko I, Kantor I, Marini C, Wilke M, Ismailova L, Solopova N, Chumakov A, Pascarelli S, Dubrovinsky L (2015) High-pressure spectroscopic study of Siderite (FeCO3) with focus on spin crossover. Am Mineral 100:2670–2681
Chaney J, Santillán JD, Knittle E, Williams Q (2014) A high-pressure infrared and Raman spectroscopic study of BaCO3: the aragonite, trigonal and Pmmn structures. Phys Chem Miner 42(1):83–93
Datchi F, Dewaele A, Loubeyre P, Letoullec R, Le Godec Y, Canny B (2007) Optical pressure sensors for high-pressure-high-temperature studies in a diamond anvil cell. High Press Res 27:447–463
Farfan G, Wang S, Ma H, Caracas R, Mao WL (2012) Bonding and structural changes in siderite at high pressure. Am Mineral 97(8–9):1421–1426
Gillet P, Biellmann C, Reynard B, McMillan P (1993) Raman spectroscopic studies of carbonates part I: high-pressure and high-temperature behaviour of calcite, magnesite, dolomite and aragonite. Phys Chem Miner 20:1–18
Gonze X, Lee C (1997) Dynamical matrices, Born effective charges, dielectric permittivity tensors, and interatomic force constants from density-functional perturbation theory. Phys Rev B 55:10355–10368
Gonze X, Amadon B, Anglade PM, Beuken JM, Bottin F, Boulanger P, Bruneval F, Caliste D, Caracas R, Cote M, Deutsch T, Genovese L, Ghosez P, Giantomassi M, Goedecker S, Hamann DR, Hermet P, Jollet F, Jomard G, Leroux S, Mancini M, Mazevet S, Oliveira MJT, Onida G, Pouillon Y, Rangel T, Rignanese GM, Sangalli D, Shaltaf R, Torrent M, Verstraete MJ, Zerah G, Zwanziger JW (2009) ABINIT: First-principles approach of materials and nanosystem properties. Comput Phys Comm 180:2582–2615
Isshiki M, Irifune T, Hirose K, Ono S, Ohishi Y, Watanuki T, Nishibori E, Takata M, Sakata M (2004) Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427(6969):60–63
Jahn S, Kowalski PM (2014) Theoretical approaches to structure and spectroscopy of Earth materials. Rev Mineral Geochem 78:691–743
Jamieson JC (1957) Introductory studies of high-pressure polymorphism to 24,000 bars by x-ray diffraction by some comments on calcite II. J Geol 65:334–343
Jing Q, Wu Q, Liu Y, Zhang Y, Liu S, Liu L, J-a Xu, Bi Y (2013) Effect of pressure and temperature on the wavelength shift of the fluorescence line of SrB4O7:Sm2+ scale. High Press Res 33(4):725–733
Koch-Müller M, Speziale S, Deon F, Mrosko M, Schade U (2011) Stress-induced proton disorder in hydrous ringwoodite. Phys Chem Miner 38(1):65–73
Koch-Müller M, Mrosko M, Gottschalk M, Schade U (2012) Pressure-induced phase transitions in ilvaite studied by in situ micro-FTIR spectroscopy. Eur J Mineral 24(5):831–838
Kraft S, Knittle E, Williams Q (1991) Carbonate stability in the Earth’s mantle: a vibrational spectroscopic study of aragonite and dolomite at high pressures and temperatures. J Geophys Res 96(B11):17997–18009
Kulshreshtha C, Cho SH, Jung YS, Sohn K (2006) Deep red color emission in an Sm2+-doped SrB4O7 phosphor. Proc ASID 6:294–296
Lin CC, Liu LG (1997) High pressure phase transformations in aragonite-type carbonates. Phys Chem Miner 24:149–157
Liu LG, Mernagh TP (1990) Phase transitions and Raman spectra of calcite at high pressures and room temperature. Am Mineral 75:801–806
Mao HK, Xu J, Bell PM (1986) Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–4676
Merlini M, Hanfland M, Crichton WA (2012) CaCO3-III and CaCO3-VI, high-pressure polymorphs of calcite: possible host structures for carbon in the Earth’s mantle. Earth Planet Sci Lett 333–334:265–271
Merlini M, Crichton WA, Chantel J, Guignard J, Poli S (2014) Evidence of interspersed co-existing CaCO3-III and CaCO3-IIIb structures in polycrystalline CaCO3 at high pressure. Mineral Mag 78(2):225–233
Merrill L, Bassett WA (1975) The high-pressure structure of CaCO3 (II), a high pressure metastable phase of calcium carbonate. Acta Cryst B31:343–349
Minch R, Dubrovinsky L, Kurnosov A, Ehm L, Knorr K, Depmeier W (2010a) Raman spectroscopic study of PbCO3 at high pressures and temperatures. Phys Chem Miner 37(1):45–56
Minch R, Seoung DH, Ehm L, Winkler B, Knorr K, Peters L, Borkowski LA, Parise JB, Lee Y, Dubrovinsky L, Depmeier W (2010b) High-pressure behavior of otavite (CdCO3). J Alloy Compd 508(2):251–257
Mirwald P (1976) A differential thermal analysis study of the high-temperature polymorphism of calcite at high pressure. Contrib Miner Petrol 59:33–40
Mrosko M, Koch-Müller M, Schade U (2011) In-situ mid/far micro-FTIR spectroscopy to trace pressure-induced phase transitions in strontium feldspar and wadsleyite. Am Mineral 96(11–12):1748–1759
Oganov AR, Glass CW, Ono S (2006) High-pressure phases of CaCO3: crystal structure prediction and experiment. Earth Planet Sci Lett 241(1–2):95–103
Oganov AR, Ono S, Ma Y, Glass CW, Garcia A (2008) Novel high-pressure structures of MgCO3, CaCO3 and CO2 and their role in Earth’s lower mantle. Earth Planet Sci Lett 273(1–2):38–47
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249
Perdew JP, Zunger A (1981) Self-interaction correction to density-functional approximations for many-electron systems. Phys Rev B 23:5048–5079
Pippinger T, Miletich R, Merlini M, Lotti P, Schouwink P, Yagi T, Crichton WA, Hanfland M (2015) Puzzling calcite-III dimorphism: crystallography, high-pressure behavior, and pathway of single-crystal transitions. Phys Chem Miner 42(1):29–43
Raju SV, Zaug JM, Chen B, Yan J, Knight JW, Jeanloz R, Clark SM (2011) Determination of the variation of the fluorescence line positions of ruby, strontium tetraborate, alexandrite, and samarium-doped yttrium aluminum garnet with pressure and temperature. J Appl Phys 110(2):023521–023528
Santillán J (2003) Dolomite-II: a high-pressure polymorph of CaMg(CO3)2. Geophys Res Lett 30(2):1054. doi:10.1029/2002gl016018
Santillán J, Williams Q (2004) A high-pressure infrared and X-ray study of FeCO3 and MnCO3; comparison with CaMg(CO3)2-dolomite. Phys Earth Planet Inter 143–144:291–304
Santillán J, Catalli K, Williams Q (2005) An infrared study of carbon-oxygen bonding in magnesite to 60 GPa. Am Mineral 90:1669–1673
Spivak A, Solopova M, Cerantola V, Bykova E, Zakharchenko E, Dubrovinsky L, Litvin Y (2014) Phys Chem Miner 41:633–638
Suito K, Namba J, Horikawa T, Taniguchi Y, Sakurai N, Kobayashi M, Onodera A, Shimomura O, Kikegawa T (2001) Phase relations of CaCO3 at high pressure and high temperature. Am Mineral 86(9):997–1002
Tyburczy JA, Ahrens TJ (1986) Dynamic compression and volatile release of carbonates. J Geophys Res 91(B5):4730–4744
Valenzano L, Noel Y, Orlando R, Zicovich-Wilson CM, Ferrero M, Dovesi R (2007) Ab initio vibrational spectra and dielectric properties of carbonates: magnesite, calcite and dolomite. Theor Chem Acc 117:991–1000
Veithen M, Gonze X, Ghosez P (2005) Nonlinear optical susceptibilities, Raman efficiencies, and electrooptic tensors from first-principles density functional perturbation theory. Phys Rev B 71:125107
Wenk H-R, Bulakh A (2004) Minerals. Their constitution and origin. Cambridge University Press, Cambridge. ISBN 0 521 82238 6
White WB (1974) The carbonate minerals. In: Farmer VC (ed) The infrared spectra of minerals. Mineralogical Society Monograph, London, pp 227–279
Williams Q, Collerson B, Knittle E (1992) Vibrational spectra of magnesite (MgCO3) and calcite III at high-pressures. Am Mineral 77:1158–1165
Wittlinger J et al (1997) High-pressure study of h.c.p.-argon. Acta Crystallogr A B53:745–749
Acknowledgments
We thank Reiner Schulz and Andreas Ebert for technical support. We thank HZB for the allocation of synchrotron radiation beamtime. This study was partly supported by a Grant from Deutsche Forschungsgemeinschaft within the Research Unit FOR2125 under Grant KO1260/16. Part of the simulations was performed at the supercomputer JUROPA at Jülich Supercomputing Centre (JSC) within the framework of NIC Grant HPO15. We thank two anonymous reviewers for their very useful comments and suggestions, which helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koch-Müller, M., Jahn, S., Birkholz, N. et al. Phase transitions in the system CaCO3 at high P and T determined by in situ vibrational spectroscopy in diamond anvil cells and first-principles simulations. Phys Chem Minerals 43, 545–561 (2016). https://doi.org/10.1007/s00269-016-0815-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-016-0815-8