Abstract
Background
Several North American studies have observed survival benefit in patients exposed to β-blockers following traumatic brain injury (TBI). The purpose of this study was to evaluate the effect of β-blockade on mortality in a Swedish cohort of isolated severe TBI patients.
Methods
The trauma registry of an urban academic trauma center was queried to identify patients with an isolated severe TBI between 1/2007 and 12/2011. Isolated severe TBI was defined as an intracranial injury with an Abbreviated Injury Scale (AIS) ≥3 excluding extra-cranial injuries AIS ≥3. Multivariable logistic regression analysis was used to determine the effect of β-blocker exposure on mortality. Also, a subgroup analysis was performed to investigate the risk of mortality in patients on pre-admission β-blocker versus not and the effect of specific type of β-blocker on the overall outcome.
Results
Overall, 874 patients met the study criteria. Of these, 33 % (n = 287) were exposed to β-blockers during their hospital admission. The exposed patients were older (62 ± 16 years vs. 49 ± 21 years, p < 0.001), and more severely injured based on their admission GCS, ISS, and head AIS scores (GCS ≤8: 32 % vs. 28 %, p = 0.007; ISS ≥16: 71 % vs. 59 %, p = 0.001; head AIS ≥4: 60 % vs. 45 %, p < 0.001). The crude mortality was higher in patients who did not receive β-blockers (17 % vs. 11 %, p = 0.007) during their admission. After adjustment for significant confounders, the patients not exposed to β-blockers had a 5-fold increased risk of in-hospital mortality (AOR 5.0, CI 95 % 2.7–8.5, p = 0.001). No difference in survival was noted in regards to the type of β-blocker used. Subgroup analysis revealed a higher risk of mortality in patients naive to β-blockers compared to those on pre-admission β-blocker therapy (AOR 3.0 CI 95 % 1.2–7.1, p = 0.015).
Conclusions
β-blocker exposure after isolated severe traumatic brain injury is associated with significantly improved survival. We also noted decreased mortality in patients on pre-admission β-blocker therapy compared to patients naive to such treatment. Further prospective studies are warranted.
Similar content being viewed by others
Introduction
Despite preventive efforts, intense research, advancements in critical care, and construction of evidence-based management guidelines by the Brain Trauma Foundation, the mortality after traumatic brain injury (TBI) continues to be the leading cause of death and disability among children and adults between ages of 1–44 years. [1–6] Recently, however, several retrospective observational studies from North-America have detected a survival benefit in patients exposed to β-blockers during their hospital admission following TBI. [7–12] This finding has been attributed to the impeding effect of β-blockers on the detrimental sympathetic hyperactivity and catecholamine surge that occurs after severe TBI. [13–16] It has been postulated that this TBI-associated hyperadrenergic state may result in non-neurological organ dysfunctions that accounts for a substantial number of TBI-related deaths. [17–22].
We aimed to investigate the effects of β-blocker exposure in a Swedish population suffering from isolated severe traumatic brain injury and we hypothesized that β-blocker exposure following severe TBI results in improved survival.
Patients and methods
The current study is a retrospective, observational cohort investigation. After IRB approval, the trauma registry of Karolinska University Hospital, an academic urban trauma center in Stockholm, Sweden, was queried for patients admitted between 1/2007 and 12/2011 with a TBI using International Classification of Disease-10 (ICD-10) codes: S06.1 to S06.9 (intracranial injuries). All adult patients (age ≥18 years) admitted with a severe isolated TBI caused by blunt trauma were enrolled. Isolated severe TBI was defined as a head Abbreviated Injury Scale score (AIS) ≥3 with chest, abdomen, and extremity AIS scores ≤2. Patients not suffering from an isolated severe TBI or those who had a head AIS = 6 were excluded. Patient data abstracted from the institutional trauma registry and patient electronic health records included age, gender, admission Glasgow Coma Scale (GCS) score, admission systolic blood pressure (SBP), intracranial injury characteristics detected on multidetector computed tomography (CT), or magnetic resonance imaging when diffuse axonal injury (DAI) was suspected, AIS for all body regions, Injury Severity Score (ISS), β-blocker exposure, the time and the type of β-blocker administered (ATC: C07A), neurosurgical interventions, intensive care unit (ICU) length of stay (LOS), hospital LOS, and in-hospital mortality.
At our institution, we adhere to the guidelines set forward by the Brain Trauma Foundation. Patients suffering from severe TBI are intubated, mechanically ventilated, and sedated. Mass lesions are evacuated as deemed appropriate by neurosurgeons. Mean arterial pressure (MAP) is measured invasively, commonly in the radial artery. Intracranial pressure (ICP) monitoring guided the therapy, which is targeted at ≤20 mmHg. Central perfusion pressure (CPP) is targeted at 50–70 mmHg, where CPP is calculated as MAP − ICP. If necessary, β-blockade therapy is utilized to reduce a potentially harmful hypertension with a goal of systolic arterial blood pressure between 100–160 mmHg, or to adjust CPP. Patients with pre-admission β-blockade therapy are started on their prescribed medication as soon as deemed medically safe by an attending physician.
Statistical analysis
The primary outcome of the study was in-hospital mortality. Demographics and clinical characteristics were compared between patients exposed versus not exposed to β-blockers during their hospital admission using bivariate analysis. For analysis purposes, several continuous variables were dichotomized using clinically relevant cut-points (age ≥55 vs. <55 years, SBP ≥90 vs. <90 mmHg, GCS >8 vs. ≤8, head AIS = 3 vs. ≥4, and ISS <15 vs. ≥16). Chi square or two-sided Fisher’s exact test was used for comparison of categorical variables, while Student t test or Mann–Whitney U test was deployed for comparison of continuous variables when appropriate. Values are reported as mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables.
Risk factors that had a p value less than 0.2 from bivariate analysis were selected for stepwise logistic regression to identify independent predictors of mortality. For assessing the effect of β-blockers on mortality, a multivariate logistic analysis was used where the study population was stratified by β-blocker exposure and adjusted for significant differences (p < 0.05) between the groups. The adjusted odds ratio (AOR) and 95 % confidence intervals (CI) were derived.
Data were entered into a computerized spreadsheet and analyzed using the Statistical Package for Social Science (SPSS 14.0 for Windows, Inc. Chicago, IL).
Results
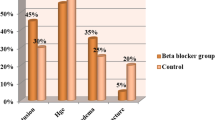
A total of 874 patients met the study inclusion criteria. Most head injuries were caused by falls (67 %), followed by motor vehicle crashes or auto versus pedestrian accidents (20 %), and assaults (13 %). Overall, 33 % (n = 287) of the study population was exposed to β-blockers during their hospital stay. When comparing the demographics and clinical characteristics between the study cohorts, patients receiving β-blockers were significantly older (62 ± 16 years vs. 49 ± 21 years, p < 0.001), and had a more severe head injury based on admission GCS, ISS, and head AIS (GCS ≤8: 32 % vs. 28 %, p = 0.007; ISS ≥16: 71 % vs. 59 %, p < 0.001; head AIS ≥4: 60 % vs. 45 %, p < 0.001). Subdural hemorrhage (45 % vs. 34 %, p = 0.002), subarachnoid hemorrhage (12 % vs. 7 %, p = 0.02), and diffuse axonal injury (4.9 % vs. 1.4 %, p = 0.002) were more frequently present in the β-blocker group compared to their non β-blocker counterparts. Patients exposed to β-blockers were less likely to undergo neurosurgical intervention (craniotomy/craniectomy: 11 % vs. 24 %, p = 0.001) (Table 1). The crude mortality was higher in patients who did not receive β-blockers (17 % vs. 11 %, p = 0.007), while both ICU and hospital LOS were extended in the β-blocker cohort (Table 2).
Bivariate analysis identified 9 factors to be potentially associated with mortality (p < 0.2), including the use of β-blockers (Table 3). These factors were entered into a stepwise logistic regression model.
As depicted in Table 4, age, hypotension on admission, the severity of TBI based on admission GCS and head AIS, subarachnoid hemorrhage, the refrain of neurosurgical intervention, and the absence of β-blocker exposure (AOR 4.6, 95 % CI 2.8–7.7, p < 0.001) were all independent predictors of mortality (Table 4).
After adjusting for differences between the cohorts, there was a 5-fold increased risk for in-hospital mortality in patients not exposed to β-blockers (AOR 5.0, 95 % CI 2.7–8.5, p = 0.001) (Table 5).
The median time to initiation of β-blocker treatment was 1 day (25th and 75th percentile:1 and 3 days)], with majority (75 %) of the treatments commenced before day 3 of admission. The two most common β-blockers used were labetalol and metoprolol in 49 % and 45 % of the population, respectively. There was no statistical difference in mortality when comparing the type of β-blocker used.
A total of 134 (46 %) patients receiving β-blockers during their admission were on β-blocker therapy prior to their admission. When comparing patients who were on β-blockers prior to their admission to those who commenced their β-blocker medication at the hospital, the pre-admission β-blocker group was significantly older (67 ± 12 years vs. 59 ± 18, p < 0.001) but less severely injured based on admission GCS, ISS, and head AIS (GCS ≤8: 31 % vs. 43 %, p = 0.03; ISS ≥16: 60 % vs. 80 %, p < 0.001; head AIS ≥4: 47 % vs. 71 %, p < 0.001), and less likely to require a neurosurgical intervention (craniectomy/craniotomy: 12 % vs. 33 %, p < 0.001) (Table 6).
After adjustment for the differences between the pre-admission versus first time in-hospital β-blocker exposed groups, there was a 3-fold (AOR 3.0, 95 % CI 1.2–7.1, p = 0.015) increased risk of mortality among patients who were naive to β-blockers prior to their traumatic insult. Likewise, there was a threefold (AOR 2.6, 95 % CI 1.4–4.9, p = 0.003) increased risk for mortality in patients never exposed to β-blockers during their hospital stay compared to the “first time in-hospital” exposed β-blocker cohort.
Discussion
Brain injury is the leading cause of death among trauma patients who arrive alive to trauma centers [2, 4, 5]. A significant proportion of these deaths are unpreventable as a result of the devastating primary brain injury. However, among those patients who survive their initial injury and hospitalization many are at risk for developing non-neurological organ dysfunctions including cardiac arrhythmias, cardiac muscle necrosis, pulmonary hypertension, neurogenic pulmonary edema, and immunosuppression with an increased risk of mortality [17, 20, 22–24]. Previous investigators have linked these extra-cranial manifestations to the catecholamine surge that accompanies severe TBI. Interestingly, this hyperadrenergic state seems to be less pronounced in severely injured trauma victims lacking intracranial injuries. [25] The hyperadrenergic state may also worsen the intracranial injury by intracerebral vasoconstriction and subsequent hypoperfusion and hypoxia of the injured brain. [26–28] Nearly three decades ago, Neil-Dwyer and colleagues published their pioneering work on hyperadrenergic state associated with nontraumatic intracranial hemorrhage that has been replicated and validated by several other investigators in patients suffering severe TBI [13, 14, 29, 30]. The TBI-associated hyperadrenergic state with elevated levels of plasma catecholamines proportional to the degree of the intracranial injury seems to occur most significantly during the first week following the traumatic insult [13–15, 19, 20, 25]. β-blockers are used in some specific TBI therapies, notably the “Lund-concept”, which is a recognized method of ICP-targeted management with an aim to restore normal intracranial physiology after TBI [31]. The “Lund- concept” argues that the best way to reabsorb cerebral edema is to control osmotic and hydrostatic differences, utilizing a combination pharmacotherapy involving β1-antagonist (metoprolol), α2-agonist (clonidine), sedation, dihydroergotamine, and maintenance of colloid osmotic pressure. This type of treatment has been shown to improve outcome compared to other types of ICP management regimes [32]. The exact beneficial effects of the Lund-concept, and in particular the role of β-blocker, have not been analyzed on a deeper level.
Recently, several retrospective clinical studies from North America have shown positive effects of in-hospital β-blocker exposure on the overall outcome after TBI [7–12, 18]. In our current investigation, we observed similar overall incidence of β-blocker exposure in head-injured patients and we noted, likewise, positive results on survival in our studied population. Nevertheless, there are several differences between our study and the studies originating from North America. Firstly, our β-blocker exposed patients were older than the North American β-blocked cohorts. We also report the prevalence of patients on β-blockers prior to their traumatic insult, as well as the type of β-blockers used. Finally, only 15 % of the Swedish population is born of foreign nationality [33], making the ethnic background more homogenous in Sweden compared to the North American settings. Overall, North American emergency departments care for a more diverse ethnic population compared to their Swedish counterparts. [12, 33–37] The effect of ethnicity on the overall outcome after TBI has been previously investigated revealing quantifiable discrepancies between diverse ethnic groups [34, 35, 37]. Likewise, the efficacy of β-blockers on survival after TBI in different ethnic groups has been noted by Bukur et al., suggesting that β-blocker exposure after TBI may not benefit all ethnic groups equally [12].
Although the protective effect of β-blockers following head injury is unclear, it is postulated that β-blockers mitigate the TBI-related hyperadrenergic state thus protecting against the extra-cranial organ dysfunction. Cotton and colleagues studied the effect of β-blocker exposure in patients who were hospitalized between 4 and 30 days for severe head injury. A total of 420 patients were included in this retrospective cohort study with 174 patients being exposed to β-blockers. Despite being of older age, more severely injured, having a higher rate of concomitant respiratory and infectious complications with a longer average length of stay, patients who had been exposed to β-blockers had a significant mortality reduction when compared to their non β-blocked counterparts (5.1 % vs. 10.8 %, p = 0.036). After adjusting for potential confounders between the cohorts, the relative risk of death between patients exposed to β-blockers and those who were not was 0.29 (95 % CI 0.14–0.6, p = 0.001) [9]. Likewise, Arbabi et al. observed a reduction in mortality after TBI in patients who had been exposed to β-blockers during their hospital admission, even though these victims of head injury were older, more severely injured, and had higher incidence of comorbidities compared to their non β-blocked counterparts. [8] Also, Inaba and colleagues observed a marked reduction in mortality in their elderly patients who had suffered a severe head injury (AIS ≥4) and had been exposed to β-blockers during their ICU stay [7]. Finally, Schroeppel and co-authors found a significant reduction in mortality (AOR 0.35, 95 % CI 0.25–0.49) in blunt TBI patients who had been exposed to β-blockers despite being older and more severely injured [10]. These investigators suggested that this survival benefit might occur due to the protective effects mediated by beta-blockers on the toxic hyperadrenergic state. Similar to the North American studies, patients in our investigation being exposed to β-blockers were significantly older and more severely injured. Despite these disadvantages seen in the β-blocker cohort, patients not exposed to β-blockers during their admission had an increased risk for mortality (OR 1.8, 95 % CI 1.2–2.7, p = 0.007). The risk for mortality was even greater after adjusting for differences between the cohorts (AOR 5.0, 95 % CI 2.7–8.5, p = 0.001). These observations support the hypothesis that β-blockade provides a positive effect on outcome, and this effect might be a result of protection against the catecholamine surge and the subsequent extra-cranial organ dysfunctions seen in patients of traumatic brain injury across the populations of North America and Scandinavia.
The timing for β-blockade therapy to mitigate the detrimental effects of the TBI-associated catecholamine surge has yet to be defined. Currently, there are no useful tests that predict or diagnose the hyperadrenergic state in clinical settings. The catecholamine surge may result in hyperthermia, tachycardia, tachypnea, diaphoresis, and hypertension, but other underlying causes should be ruled out first [18]. At our institution, patients receive β-blockade agents as first line of therapy to reduce potentially harmful hypertensive episodes and for maintaining a steady central perfusion pressure to avoid the risk of cerebral hyperemia [38, 39]. When comparing patients who were on pre-admission β-blocker therapy to those who had no such treatment pre-injury, we noticed a significant difference in the timing of first dose β-blocker administered. The median time for initiation of β-blocker therapy was 1 day (25th and 75th percentile:1 and 2 days) in patients who were on β-blockers and 1 day (25th and 75th percentile: 1 and 6 days) in patients not on β-blockers prior to their injury. An explanation for this finding could be the in-hospital reconciliation with patients’ home medications rather than treatment of a complication. After adjusting for differences between patients on β-blockers prior to their admission with those who were exposed to β-blockers for the first time in-hospital, we noted a 3-fold increased risk for mortality (AOR 3.0, 95 % CI 1.2–7.1, p = 0.002) in the latter group. This finding suggests that early and continuous β-blockade may down-regulate the detrimental hyperadrenergic state that is associated with severe TBI.
The main limitation of this study inherited to its retrospective design. Secondly, we were not able to control for comorbidities in our study population, neither do we know the exact cause of death for the TBI-treated patients in our study. Thirdly, we may have introduced a potential treatment bias where β-blockade therapy is administered only in cases where survival is deemed possible. Also, the results might reflect a social difference since patients with β-blockade therapy might be on a higher socio-economic level and thus have lower co-morbidity, and probably a better outcome, than patients in lower socio-economic groups. Although our study does support the theory of the beneficial effects of β-blocker exposure in TBI patients, it does not answer the questions regarding the timing of intervention and duration of therapy. Finally, our data only prove a survival benefit, however, does not explain the mechanism of the outcome benefit nor the quality of life for the surviving patients.
Due to the retrospective design of our investigation and previous studies on the topic, we do recommend that these results should be interpreted with caution until verified by prospective randomized controlled investigations.
Conclusion
Our study reports that β-blocker exposure in patients sustaining an isolated severe TBI is associated with improved survival. Prospective evaluation of our results is warranted.
References
Thurman D (2001) The epidemiology and economics of head trauma. In: Miller L, Hayes R (eds) Head trauma: basic, preclinical, and clinical directions. Wiley, New York
Shackford SR, Mackersie RC, Holbrook TL et al (1993) The epidemiology of traumatic death. A population-based analysis. Arch Surg 128(5):571–575
Corrigan JD, Selassie AW, Orman JA (2010) The epidemiology of traumatic brain injury. J Head Trauma Rehabil 25(2):72–80
Cales RH, Trunkey DD (1985) Preventable trauma deaths. A review of trauma care systems development. JAMA 254(8):1059–1063
Acosta JA, Yang JC, Winchell RJ et al (1998) Lethal injuries and time to death in a level I trauma center. J Am Coll Surg 186(5):528–533
Brain Trauma Foundation (2007) American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 24(Suppl 1):S1–106
Inaba K, Teixeira PG, David JS et al (2008) Beta-blockers in isolated blunt head injury. J Am Coll Surg 206(3):432–438. doi:10.1016/j.jamcollsurg.2007.10.005
Arbabi S, Campion EM, Hemmila MR et al (2007) Beta-blocker use is associated with improved outcomes in adult trauma patients. J Trauma 62(1):56–61; discussion 61-2
Cotton BA, Snodgrass KB, Fleming SB et al (2007) Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma 62(1):26–33; discussion 33-5
Schroeppel TJ, Fischer PE, Zarzaur BL et al (2010) Beta-adrenergic blockade and traumatic brain injury: protective? J Trauma 69(4):776–782
Schroeppel TJ, Sharpe JP, Magnotti LJ et al (2014) Traumatic brain injury and beta-blockers: not all drugs are created equal. J Trauma Acute Care Surg 76(2):504–509
Bukur M, Mohseni S, Ley E et al (2012) Efficacy of beta-blockade after isolated blunt head injury: does race matter? J Trauma Acute Care Surg 72(4):1013–1018
Hortnagl H, Hammerle AF, Hackl JM, Brucke T, Rumpl E, Hortnagl H (1980) The activity of the sympathetic nervous system following severe head injury. Intensive Care Med 6(3):169-1-7
Clifton GL, Ziegler MG, Grossman RG (1981) Circulating catecholamines and sympathetic activity after head injury. Neurosurgery 8(1):10–14
Hamill RW, Woolf PD, McDonald JV, Lee LA, Kelly M (1987) Catecholamines predict outcome in traumatic brain injury. Ann Neurol 21(5):438–443
Liu MY (1995) Protective effects of propranolol on experimentally head-injured mouse brains. J Formos Med Assoc 94(7):386–390
Kemp CD, Johnson JC, Riordan WP, Cotton BA (2008) How we die: the impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am Surg 74(9):866–872
Heffernan DS, Inaba K, Arbabi S, Cotton BA (2010) Sympathetic hyperactivity after traumatic brain injury and the role of beta-blocker therapy. J Trauma 69(6):1602–1609
Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ (2005) Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med 33(3):654–660
Piek J, Chesnut RM, Marshall LF et al (1992) Extracranial complications of severe head injury. J Neurosurg 77(6):901–907
Grunsfeld A, Fletcher JJ, Nathan BR (2005) Cardiopulmonary complications of brain injury. Curr Neurol Neurosci Rep 5(6):488–493
Clifton GL, Robertson CS, Kyper K, Taylor AA, Dhekne RD, Grossman RG (1983) Cardiovascular response to severe head injury. J Neurosurg 59(3):447–454
Clifton GL, Robertson CS, Grossman RG (1989) Cardiovascular and metabolic responses to severe head injury. Neurosurg Rev 12(Suppl 1):465–473
Woiciechowsky C, Asadullah K, Nestler D et al (1998) Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med 4(7):808–813
Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C (1992) The catecholamine response to multisystem trauma. Arch Surg 127(8):899–903
Alexander RW, Davis JN, Lefkowitz RJ (1975) Direct identification and characterisation of beta-adrenergic receptors in rat brain. Nature 258(5534):437–440
MacKenzie ET, McCulloch J, Harper AM (1976) Influence of endogenous norepinephrine on cerebral blood flow and metabolism. Am J Physiol 231(2):489–494
Meyer JS, Miyakawa Y, Welch KM et al (1976) Influence of adrenergic receptor blockade on circulatory and metabolic effects of disordered neurotransmitter function in stroke patients. Stroke 7(2):158–167
Neil-Dwyer G, Cruickshank JM, Doshi R (1990) The stress response in subarachnoid haemorrhage and head injury. Acta Neurochir Suppl (Wien) 47:102–110
Naredi S, Lambert G, Eden E et al (2000) Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke 31(4):901–906
Asgeirsson B, Grande PO, Nordstrom CH (1994) A new therapy of post-trauma brain oedema based on haemodynamic principles for brain volume regulation. Intensive Care Med 20(4):260–267
Eker C, Asgeirsson B, Grande PO, Schalen W, Nordstrom CH (1998) Improved outcome after severe head injury with a new therapy based on principles for brain volume regulation and preserved microcirculation. Crit Care Med 26(11):1881–1886
www.SCB.se. Statistics Sweden, data acquired 2014-01-04
Kraus JF, Fife D, Ramstein K, Conroy C, Cox P (1986) The relationship of family income to the incidence, external causes, and outcomes of serious brain injury, san diego county, california. Am J Public Health 76(11):1345–1347
Sorani MD, Lee M, Kim H, Meeker M, Manley GT (2009) Race\ethnicity and outcome after traumatic brain injury at a single, diverse center. J Trauma 67(1):75–80
Jager TE, Weiss HB, Coben JH, Pepe PE (2000) Traumatic brain injuries evaluated in U.S. emergency departments, 1992–1994. Acad Emerg Med 7(2):134–140
Bowman SM, Martin DP, Sharar SR, Zimmerman FJ (2007) Racial disparities in outcomes of persons with moderate to severe traumatic brain injury. Med Care 45(7):686–690
Mayer SA, Kurtz P, Wyman A et al (2011) Clinical practices, complications, and mortality in neurological patients with acute severe hypertension: the studying the treatment of acute hyperTension registry. Crit Care Med 39(10):2330–2336
Rose JC, Mayer SA (2004) Optimizing blood pressure in neurological emergencies. Neurocrit Care 1(3):287–299
Conflict of interest
The authors declare no conflict of interest with regard to this manuscript. No Competing financial interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohseni, S., Talving, P., Thelin, E.P. et al. The Effect of β-blockade on Survival After Isolated Severe Traumatic Brain Injury. World J Surg 39, 2076–2083 (2015). https://doi.org/10.1007/s00268-015-3039-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3039-z