Abstract
Background
Human acellular dermal matrices (ADMs) have enabled successful breast reconstructions while decreasing muscle donor morbidity and pain for the patient. However, some literature reports indicate an increase in complications, especially infection. The decellularization and terminal sterilization properties of DermACELL (D-ADM), a human ADM, may reduce the rate of complications in augmented breast reconstruction while still maintaining successful outcomes. In the study presented here, we evaluate the quality and safety of outcomes with the use of D-ADM during tissue expander breast reconstruction.
Methods
A retrospective chart review was conducted of patients who underwent breast reconstruction with the use of D-ADM, at a single-military hospital-based practice, resulting in a population of 38 subjects and 58 breasts who had breast reconstruction augmented with D-ADM.
Results
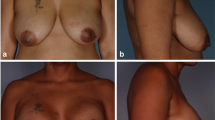
Fifty-six breasts (96.6%) in thirty-six patients demonstrated successful outcomes with a median 27 weeks’ time to complete healing. Post-reconstruction radiation and chemotherapy were applied to 24.1 and 25.9% of reconstructions, respectively. Complications rates were minimal with rates of 1.7% for surgical site infection and 1.7% for red breast syndrome.
Conclusion
The low complication rates combined with the high success and patient satisfaction rates observed for D-ADM support the use of this ADM in breast reconstruction.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these evidence-based medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.





Similar content being viewed by others
References
Antony AK, McCarthy CM, Cordeiro PG, Mehrara BJ, Pusic AL, Teo EH, Arriaga AF, Disa JJ (2010) Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 125(6):1606–1614
Chun YS, Verma K, Rosen H, Lipsitz S, Morris D, Kenney P, Eriksson E (2010) Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 125(2):429–436
Kim JY, Davila AA, Persing S, Connor CM, Jovanovic B, Khan SA, Fine N, Rawlani V (2012) A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 129(1):28–41
Salzberg CA (2006) Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 57(1):1–5
Lanier ST, Wang ED, Chen JJ, Arora BP, Katz SM, Gelfand MA, Khan SU, Dagum AB, Bui DT (2010) The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 64(5):674–678
Sbitany H, Sandeen SN, Amalfi AN, Davenport MS, Langstein HN (2009) Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 124(6):1735–1740
Liu AS, Kao HK, Reish RG, Hergrueter CA, May JW Jr, Guo L (2011) Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg 127(5):1755–1762
Brooke S, Mesa J, Uluer M, Michelotti B, Moyer K, Neves RI, Mackay D, Potochny J (2012) Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings. Ann Plast Surg 69(4):347–349
Yuen JC, Yue CJ, Erickson SW, Cooper S, Boneti C, Henry-Tillman R, Klimberg S (2014) Comparison between freeze-dried and ready-to-use AlloDerm in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open 2(3):e119
Buseman J, Wong L, Kemper P, Hill JL, Nimtz J, Rinker B, Vasconez HC (2013) Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg 70(5):497–499
Weichman KE, Wilson SC, Saadeh PB, Hazen A, Levine JP, Choi M, Karp NS (2013) Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg 132(4):725–736
Moore MA, Samsell B, Wallis G, Triplett S, Chen S, Jones AL, Qin X (2015) Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review. Cell Tissue Bank 16(2):249–259
Nahabedian MY (2009) AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 124(6):1743–1753
Newman MI, Hanabergh E, Samson MC (2010) AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 126(3):1120; author reply 1120–1121
Weichman KE, Wilson SC, Weinstein AL, Hazen A, Levine JP, Choi M, Karp NS (2012) The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 129(5):1049–1058
Samsell B, Moore MA (2012) Use of controlled low dose gamma irradiation to sterilize allograft tendons for ACL reconstruction: biomechanical and clinical perspective. Cell Tissue Bank 13(2):217–223
Woo A, Harless C, Jacobson SR (2015) Revisiting an old place: single surgeon experience on post-mastectomy subcutaneous implant based breast reconstruction. Plast Reconstr Surg 136(4 Suppl):83
McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, Cordeiro PG, Pusic AL (2008) Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 121(6):1886–1892
Spear SL, Seruya M, Rao SS, Rottman S, Stolle E, Cohen M, Rose KM, Parikh PM, Nahabedian MY (2012) Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 130(1):1–9
Colwell AS, Damjanovic B, Zahedi B, Medford-Davis L, Hertl C, Austen WG Jr (2011) Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg 128(6):1170–1178
Goodwin SJ, McCarthy CM, Pusic AL, Bui D, Howard M, Disa JJ, Cordeiro PG, Mehrara BJ (2005) Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg 55(1):16–19; discussion 19–20
Padubidri AN, Yetman R, Browne E, Lucas A, Papay F, Larive B, Zins J (2001) Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg 107(2):342–349; discussion 350–341
Garvey PB, Villa MT, Rozanski AT, Liu J, Robb GL, Beahm EK (2012) The advantages of free abdominal-based flaps over implants for breast reconstruction in obese patients. Plast Reconstr Surg 130(5):991–1000
Spear S, Parikh P, Reisin E, Menon NG (2008) Acellular dermis-assisted breast reconstruction. Aesthet Plast Surg 32(4):418–425
Becker S, Saint-Cyr M, Wong C, Dauwe P, Nagarkar P, Thornton JF, Peng Y (2009) AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg 123(1):1–6; discussion 107–108
Vashi C (2014) Clinical outcomes for breast cancer patients undergoing mastectomy and reconstruction with use of DermACELL, a sterile, room temperature acellular dermal matrix. Plast Surg Int 2014:1–7
Bullocks JM (2014) DermACELL: a novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur J Plast Surg 37(10):529–538
Yu D, Hanna K, LeGallo R, Drake D (2016) Comparison of histological characteristics of acellular dermal matrix capsules to surrounding breast capsules in acellular dermal matrix assisted breast reconstruction. Ann Plast Surg 16(5):485–488
Norton L, Babenesee J (2009) Innate and adaptive immune responses in tisse engineering. In: Meyer U, Meyer T, Handschel J, Wesmann HP (eds) Fundamentals of tissue engineering and regenerative medicine. Springer, Berlin, pp 721–745
Qin X, Cotter AT, Chen S, Chen J, Wolfinbarger L. Gamma-irradiated human acellular dermis: a potential treatment for wound and soft tissue defects. Paper presented at: Society for American Wound Care. 21st Annual Mtg. Apr 24-272008
Rutala WA, Weber DJ (2008) Guideline for disinfection and sterilization in healthcare facilities, 2008. Centers for Disease Control and Prevention (CDC), Atlanta
Glasberg SB, Light D (2012) AlloDerm and Strattice in breast reconstruction: a comparison of techniques for optimizing outcomes. Plast Reconstr Surg 129(6):1223–1233
Butterfield JL (2013) 440 consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg 131:940–951
Hanna KR, Tilt A, Holland M, Colen D, Bowen B, Stovall M, Lee A, Wang J, Drake D, Lin K, Uroskie T, Campbell CA (2016) Reducing infectious complications in implant based breast reconstruction: impact of early expansion and prolonged drain use. Ann Plast Surg 76(Suppl 4):S312–S315
Liu DZ, Mathes DW, Neligan PC, Said HK, Louie O (2014) Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg 72(5):503–507
Mendenhall SD, Anderson LA, Ying J, Boucher KM, Liu T, Neumayer LA, Agarwal JP (2015) The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast Reconstr Surg 135(1):29e–42e
Moyer HR, Pinell-White X, Losken A (2014) The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg 133(2):214–221
Nguyen M-D, Chen C, Colakoğlu S, Morris DJ, Tobias AM, Lee BT (2010) Infectious complications leading to explantation in implant-based breast reconstruction with AlloDerm. Eplasty 10:e48
Parks JW, Hammond SE, Walsh WA, Adams RL, Chandler RG, Luce EA (2012) Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg 130(4):739–746
Rawlani V, Buck DW 2nd, Johnson SA, Heyer KS, Kim JY (2011) Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg 66(6):593–597
Salzberg CA, Ashikari AY, Koch RM, Chabner-Thompson E (2011) An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 127(2):514–524
Seth AK, Persing S, Conner CM, Davila AA, Hirsch E, Fine NA, Kim JY (2013) A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction. Ann Plast Surg 70(6):632–635
Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, Crisera C, Festekjian JH (2011) Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 125(5):403e–410e
Venturi ML, Mesbahi AN, Boehmler JH, Marrogi AJ (2013) Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg 131(1):9e–18e
Acknowledgments
This study was funded through a grant by The Geneva Foundation, a nonprofit organization that supports and advances innovative medical research and excellence in education within the US military. No funding was provided directly to the author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No other conflicts of interest are disclosed by the author.
Rights and permissions
About this article
Cite this article
Ortiz, J.A. Clinical Outcomes in Breast Reconstruction Patients Using a Sterile Acellular Dermal Matrix Allograft. Aesth Plast Surg 41, 542–550 (2017). https://doi.org/10.1007/s00266-017-0817-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-017-0817-z