Abstract
Background
Several surgical strategies have evolved for the treatment of focal axillary hyperhidrosis (FAH). However, nonresponders are found in every procedure. Until now no characterization of the recurrent sweating areas has been reported. The aim of this study was to characterize the axillary sweat area by using the iodine starch test in nonresponders prior to surgery.
Methods
Prior to minimally invasive surgery, 24 (15 females, 9 males) nonresponders underwent a repetitive iodine starch test to define the area of recurrent sweating. Size and distribution of the hyperhidrotic area were documented.
Results
Eighteen patients had undergone previous liposuction and six liposuction curettage. The size of recurrent sweating area was 10.2 cm2 (range = 5.5–24.5 cm2). We were able to identify three different patterns of recurrent sweating: crescent (33%), circular (41%), and insertion-spot type (26%).
Conclusion
Patterns of recurrent sweating areas may partially indicate insufficient planning and implementation of surgery, resulting in nonresponders. We suggest that exact preoperative identification of the hyperhidrotic area be mandatory.
Similar content being viewed by others
Surgery is necessary for the permanent reduction of excessive focal axillary hyperhidrosis (FAH). Recently, minimally invasive strategies such as superficial liposuction, manual curettage, or liposuction curettage have been favored, and complete excision of the axillary skin is performed less frequently [1–6]. Although several techniques have been reported to be effective and beneficial to the patients’ quality of life, nonresponders with recurrent sweating remain a problem [6]. Efficacy seems to rise with the aggressiveness of the procedure, as sharp cannulas have been shown to reduce sweat rates more effectively than blunt instruments [7, 8]. However, the recurrence rate varies widely in the literature, ranging from 2% to 20% [2, 9]. The exact reason for inadequate surgery still remains unclear. Some authors believe that the minimally invasive procedure itself is incapable of producing a permanent cure for FAH, leading to only a temporary breakdown of sweat gland innervation [10, 11]. Others propose that if surgery is performed too sparingly without aggressive removal of sweat glands, only inadequate sweat reduction is possible [7, 9].
The extent and anatomical distribution of recurrent axillary sweating have not been evaluated so far. Therefore, we initiated a study to evaluate the extent and distribution patterns of axillary sweating areas in patients who had undergone inadequate minimally invasive surgery.
Materials and Methods
Patients
Twenty-four patients (15 females, 9 males; median age = 27.8 years, range = 19–42 years) with FAH were enrolled in the study. All patients had undergone unsuccessful minimally invasive surgery for FAH prior to being recruited into the present study. Eighteen had unsuccessful superficial liposuction and six had liposuction curettage. Twenty-one patients were referred to our hyperhidrosis outpatient unit from other hospitals or private clinics and three had undergone surgery in our department. Fifteen patients reported recurrent bilateral sweating, whereas nine patients indicated that only one axilla was hyperhidrotic. This resulted in 39 axillae that were included in the present study.
Iodine Starch Test
To define the area of recurrent sweating, an iodine starch test was performed. To ensure that the iodine starch test findings were reproducible, every axilla was tested three times. For the starch test we used Lugol’s solution (also known as Lugol’s iodine), i.e., iodine, 1.0 g; potassium iodide, 2.0 g; and distilled water, 100.0 ml, a modification of the original Minor formulation [12]. Before testing, the axilla was dried thoroughly, painted with the iodine tincture, then air-dried and dusted with commercial potato flour. As the patient began to sweat, moistened starch reacted with the iodine to produce a vivid blue color. The size of hyperhidrotic area was quantified by using a transparent plastic foil with standardized grids (0.5 × 0.5 cm), enabling an exact measurement of the hyperhidrotic area in square centimeters [13]. Measurement was performed 60 s after application of the potato flour.
Results
Iodine Starch Test
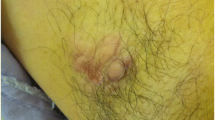
The mean size of the recurrent hyperhidrotic area was 10.2 cm2 (range = 5.5–24.5 cm2). In the iodine starch tests, three different patterns of sweat area distribution could be distinguished: the crescent type (13 axillae, 33%), the circular type (16 axillae, 41%), and the spot-like form surrounding the insertions (10 axillae; 26%) (Fig. 1a–c). The crescent type occurred on the left side in 69% of those cases, always situated along the major pectoral muscle. The circular type was found equally on both sides around the hair-bearing axillary skin. The spot-like type was not uniformly distributed on both sides (70% right, 30% left) and presented as hyperhidrotic spots, each close to the insertion points of prior surgery.
a Crescent hyperhidrotic area situated laterally along the major pectoral muscle. b Circular-like recurrent hyperhidrotic area surrounding the hair-bearing axillary center. c Spot-like sweating areas located around the insertion points of the cannula. In all photos, the vivid blue color after iodine starch test indicates the hyperhidrotic area
Discussion
For a permanent cure of axillary hyperhidrosis there are different surgical strategies reported in the literature, which can be divided primarily into local surgery and interruption techniques of the sympathic nerves (Table 1). The percentage of nonresponders to minimally invasive surgery, however, varies widely in the literature, ranging from 2% to 20% [2, 9]. Until today, the characteristics of recurrent axillary sweating and the extent of refractory hyperhidrotic areas have not been described in detail. This is due in part to inadequate methodology for evaluating the surgical outcome, making most results difficult to compare. Usually only subjective parameters such as the patient’s evaluation of symptoms are reported, whereas qualitative (e.g., iodone starch test) or quantitative (e.g., gravimetry) tests are often missing [1, 2, 4, 14].
Supporters of botulinum toxin A injections have recently expressed doubt that subcutaneous ablation of sweat glands is capable of permanent FAH reduction [10, 15]. They assume that minimally invasive strategies lead to only a temporary interruption of sweat gland innervation without removal of the glands themselves [10]. In contrast, supporters of surgical options believe that subcutaneous ablation techniques are efficient in removing sweat glands as shown by histologic studies, although inadequate surgery remains a possibility [8, 16].
A description of the recurrent sweating area could be helpful in assessing whether minimally invasive methods are inappropriate per se or whether an insufficient surgical performance might be responsible for the phenomenon of nonresponders. Interestingly, we showed that the central axillary region, which is normally the most secretory active area, did not reveal recurrent hyperhidrosis in any patient. Instead, three different patterns of recurrent sweat areas were identified: the crescent type, the circular type, and the spot-like form surrounding the insertion points of prior surgery. To explain the patterns we found, we assume that the surgeon focused mainly on the central axillary region, which is often the most hyperhidrotic area. The crescent type was always observed at the lateral aspect of the pectoralis muscle. The specific anatomical peculiarities of the peripheral axillary region could play a role in insufficient surgery. Our surgical experience confirms that the cupped depression between the pectoralis muscle and the axilla interferes with a subtle, careful curettage. Concerning the spot-like type, our surgical experience shows that the affected areas are difficult to reach by curettage, which is regularly performed in a fan-like fashion. The pressure that is necessary for the rasps of the cannula opening is not adequate around the insertion points; we feel that this is responsible for the spot-like pattern
On the one hand, our findings confirm that minimally invasive strategies are indeed capable of permanently reducing axillary hyperhidrosis. However, they also demonstrate that in the patients we studied, inadequate mapping of the FAH area and/or careless surgical technique may have occurred. Therefore, preoperative mapping of the hyperhidrotic area should be performed using an iodine starch test, followed by precise surgery of all identified sweating regions of the axillary skin.
References
Böni R (2006) Tumescent suction curettage in the treatment of axillary hyperhidrosis: experience in 63 patients. Dermatology 213:215–217
Lee D, Cho SH, Kim YC, Park JH, Lee SS, Park SW (2006) Tumescent liposuction with dermal curettage for treatment of axillary osmidrosis and hyperhidrosis. Dermatol Surg 32:505–511
Tung TC, Wei FC (1997) Excision of subcutaneous tissue for the treatment of axillary osmidrosis. Br J Plast Surg 50:61–66
Bechara FG, Sand M, Sand D, Altmeyer P, Hoffmann K (2007) Suction-curettage as a surgical treatment of focal axillary hyperhidrosis: recommendation for an aggressive approach. Plast Reconstr Surg 119:1390–1391
Definition and therapy of primary hyperhidrosis. Guidelines of the German Society of Dermatology (DDG), February 2007. Available at http://www.uni-duesseldorf.de/AWMF/ll/013-059.htm. Accessed 27 Oct 2008
Bechara FG, Gambichler T, Bader A, Sand M, Altmeyer P, Hoffmann K (2007) Assessment of quality of life in patients with primary axillary hyperhidrosis before and after suction-curettage. J Am Acad Dermatol 57:207–212
Bechara FG, Sand M, Sand D, Altmeyer P, Hoffmann K (2006) Surgical treatment of axillary hyperhidrosis: a study comparing liposuction cannulas with a suction-curettage cannula. Ann Plast Surg 56:654–657
Park YJ, Shin MS (2001) What is the best method for treating osmidrosis? Ann Plast Surg 47:303–309
Perng CK, Yeh FL, Ma H, Lin JT, Hwang CH, Shen BH, Chen CH, Fang RH (2004) Is the treatment of axillary osmidrosis with liposuction better than open surgery? Plast Reconstr Surg 114:93–97
Heckmann M, Rzany B (2002) Botulinumtoxin in der Dermatologie. Grundlagen und praktische Anwendung. Urban & Vogel, München
Lawrence CM, Lonsdale Eccles AA (2006) Selective sweat gland removal with minimal skin excision in the treatment of axillary hyperhidrosis: a retrospective clinical and histological review of 15 patients. Br J Dermatol 155:115–118
Minor V (1929) Ein neues Verfahren zu der klinischen Untersuchung der Schweißabsonderung. Dtsch Z Nervenheilk 101:302–308
Bechara FG, Sand M, Sand D, Achenbach RK, Altmeyer P, Hoffmann K (2006) Focal hyperhidrosis of the anal fold: a simple technique for diagnosis and evaluation of therapy. Br J Dermatol 155:858
Tsai RY, Lin JY (2001) Experience of tumescent liposuction in the treatment of osmidrosis. Dermatol Surg 27:446–448
Naumann M, Lowe NJ, Kumar CR, Hamm H (2003) Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol 139:731–736
Bechara FG, Sand M, Hoffmann K, Boorboor P, Altmeyer P, Stucker M (2008) Histological and clinical findings in different surgical strategies for focal axillary hyperhidrosis. Dermatol Surg 34:1001–1009
Author information
Authors and Affiliations
Corresponding author
Additional information
F. G. Bechara and M. Sand have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bechara, F.G., Sand, M. & Altmeyer, P. Characteristics of Refractory Sweating Areas Following Minimally Invasive Surgery for Axillary Hyperhidrosis. Aesth Plast Surg 33, 308–311 (2009). https://doi.org/10.1007/s00266-008-9261-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9261-4