Abstract
Alliance formation is a critical dimension of social intelligence in political, social and biological systems. As some allies may provide greater “leverage” than others during social conflict, the cognitive architecture that supports alliance formation in humans may be shaped by recent experience, for example in light of the outcomes of violent or non-violent forms intrasexual competition. Here we used experimental priming techniques to explore this issue. Consistent with our predictions, while men’s preferences for dominant allies strengthened following losses (compared to victories) in violent intrasexual contests, women’s preferences for dominant allies weakened following losses (compared to victories) in violent intrasexual contests. Our findings suggest that while men may prefer dominant (i.e. masculine) allies following losses in violent confrontation in order to facilitate successful resource competition, women may “tend and befriend” following this scenario and seek support from prosocial (i.e. feminine) allies and/or avoid the potential costs of dominant allies as long-term social partners. Moreover, they demonstrate facultative responses to signals related to dominance in allies, which may shape sex differences in sociality in light of recent experience and suggest that intrasexual selection has shaped social intelligence in humans.
Significance statement
Although alliance formation is an important facet of social intelligence in political and biological systems, we know relatively little about the cognitive processes involved in social preferences for allies. As recent experience may alter the leverage provided by different social partners, here we tested whether preferences for facial cues to dominance-prosociality (masculinity-femininity) alter in light of recent experience of violent and economic contests for status. Our findings demonstrate sex-specific responses to these facial cues. While men’s preferences for facial cues related to dominance in allies strengthen following losses (compared to wins) in violent contests, women’s preferences for facial cues related to dominance in allies weaken following losses (compared to wins) in violent contests. These findings suggest that intrasexual selection, in part, has shaped the evolution of social intelligence in humans as revealed in flexibility in social preferences for allies.
Similar content being viewed by others
Introduction
An important aspect of social intelligence is the ability to cooperate within strategic alliances in order to maximise reproductive fitness (see DeScioli and Kurzban 2009 for discussion). Many non-human species form both coalitions, where two parties simultaneously aggress against a third party, and longer-term alliances, where coalitions are revisited over time, normally against multiple opponents (see Harcourt and de Waal 1992). For example, male dolphins form both small and stable, and larger, flexible alliances with other males who, in turn, enhance their ability to compete for access to mates (e.g. Connor et al. 1999, 2001; Whitehead and Connor 2005). Moreover, male wild Guinea baboons form different levels of alliance with other males (Patzelt et al. 2014) and, in some primates, male alliances directly increase their reproductive success and dominance rank (Schülke et al. 2010; Gilby et al. 2013). In other examples, such as Camargue horses (Feh 1999) and male chimpanzees (Duffy et al. 2007), support from high-ranking partners facilitates access to mates, and, among male savannah baboons, coalitions improve fighting ability against rivals (Noë and Sluijter 1995; see also Caro and Collins 1987 and Packer and Pusey 1982 for coalitions in male cheetahs and lions). Moreover, white-faced capuchins form coalitions based on both shared affinity and the partner’s rank exceeding that of a rival (Perry et al. 2004), and ravens provide support to partners according to affinity (e.g. indexed via grooming) and the partner’s dominance rank in order to gain future agonistic support (Fraser and Bugnyar 2012). Female savannah baboons also provide aid to females in disputes (Silk et al. 2004), with longevity increasing among females, independent of dominance rank, with the provision of close social bonds (e.g. as indexed by frequent grooming and/or contact within a set time period; Silk et al. 2010). Collectively, there are potential benefits to alliances with conspecifics, including the facilitation of successful resource competition.
Humans form large and complex social networks (e.g. Hill and Dunbar 2003; Snyder 2007), with high quality social and emotional support from others having direct effects on proxies for reproductive fitness such as health indexed via longevity (see Holt-Lunstad et al. 2010 for a meta-analytic review), which, in turn, can maximise reproductive fitness over generations (e.g. raising offspring to independence; see Lawson and Mace 2011 for general discussion). Cooperative alliances may have been important for status acquisition throughout human evolution, as individuals share valued traits and expertise with one another rather than inflicting costs on them for direct access to resources, providing “leverage” (Hand 1986) during social conflict (see Henrich and Gil-White 2001). Consistent with this proposal, fossil record evidence suggests that violent male-male competition was an important factor in the evolution of human cooperation (Bowles 2009) and physical traits that denote formidability (i.e. ability to dominate in a contest) appear to be valued in leaders, in part, in order to attract group members who can resolve social conflicts more generally (i.e. also on a smaller-scale; see van Vugt and Grabo 2015 for recent discussion). Indeed, both physically dominant and prestigious men have higher fertility, more support from allies and are more likely to be deferred to by competitors (von Rueden et al. 2011), and male coalitionary aggression facilitates reproductive opportunities for males and community cohesion (Macfarlan et al. 2014). Collectively, both direct (i.e. violent) and indirect competition for resources (acquiring resources through force versus consumption and/or skill respectively, e.g. Smallegange et al. 2006) may have shaped the cognitive architecture that underpins alliance formation in humans (see also DeScioli and Kurzban 2009 for discussion).
Sexually dimorphic physical characteristics signal traits that may be sought after in allies, as they play an important role in within-sex competition (reviewed in Emlen 2008; Santos et al. 2011) and are correlated with male dominance rank (e.g. Pelletier and Festa-Bianchet 2006), fighting ability (e.g. Bergeron et al. 2010), physical strength (e.g. Malo et al. 2009) and reproductive fitness (e.g. Preston et al. 2003) in many non-human animal species. In humans, although other cues indicate a social partner’s relative dominance, such as eye gaze (Jones et al. 2010) and anger (Ackerman et al. 2006), masculine physical characteristics, such as low voice pitch, high facial width to height ratio and muscularity, are positively associated with both perceptions of dominance and measures of actual traits related to dominance (e.g. Stirrat and Perrett 2010; Petersen et al. 2013; reviewed in Puts 2010; Watkins et al. 2010a) and feminine physical characteristics, such as softer face shape and large eyes, are associated with prosocial traits such as perceived ability to provide high-quality social support (see Watkins et al. 2012a for discussion in the context of emotional support and investment). Indeed, the effects of digitally enhanced facial masculinity on dominance perceptions are substantial (see Puts 2010 for a summary) and the speed of trait judgements of faces is functionally adaptive if fast approach/avoid behaviour on these dimensions is favoured over accurate approach/avoid behaviour on identical dimensions (reviewed in Todorov et al. 2008). Collectively, in light of the benefits of avoiding costly intrasexual conflict (Puts 2010) and in selecting cooperative social partners (Queller 2011), sexually dimorphic characteristics may be utilised at minimal acquaintance in order to approach or avoid potential social partners.
While dominance and prosociality are potentially valuable traits in allies and are gauged, in part, from sexually dimorphic facial characteristics (Puts 2010; Watkins et al. 2012a), preferences for cues to these traits may be facultative and respond in light of recent experience such as one’s own success or failure in contests for status. Contest outcomes moderate engagement in further confrontation in various species (reviewed in Hsu et al. 2006). In humans, men’s perceptions of other men’s dominance alter in light of contest outcomes, such that losers of confrontations perceive facial cues of dominance (i.e. facial masculinity) to be more salient than winners do (i.e. losers are more likely to associate facial masculinity with high dominance; Watkins and Jones 2012; see also Welling et al. 2016 for further discussion of experience and competition). Recent work, however, suggests that having an ally decreases judgements of formidability in potential rivals (Fessler and Holbrook 2013), complementing work which demonstrates that support from coalition partners predicts success in dyadic conflict (von Rueden et al. 2008). Social perceptions of allies may therefore function to increase the leverage of individuals (Hand 1986) when attempting to resolve social conflicts in light of recent experience (see also DeScioli and Kurzban 2009).
Following on from priming experiments that test for effects of recent confrontations on judgements of potential rivals (Watkins and Jones 2012), here we adapt this paradigm to test for effects of recent contests for status on judgements of potential allies. Specifically, we test whether the nature of competition within the environment (direct/violent versus indirect/economic) and the outcomes of recent contests (win or loss) moderate preferences for sexually dimorphic facial characteristics in allies. We predict that sexually dimorphic cues will be preferred, on average, when men, but not women, judge other men as allies, in light of the greater fitness advantages to males who formed large groups to facilitate successful resource competition against rival groups (Bowles 2009; Benenson et al. 2013), and as men with dominance-related characteristics are better-placed to provide leverage to individuals as allies via the threat they pose to those groups (i.e. “parochial altruism”; Choi and Bowles 2007; McDonald et al. 2012).
Secondly, by manipulating masculine shape cues in faces, we predict sex-specific responses to facial cues related to dominance in allies following different outcomes and forms of resource competition. Whereas male sociality is oriented toward behaviours that facilitate successful competition for mates and/or resources (e.g. seeking instrumental support; McDonald et al. 2012; Benenson et al. 2013), female sociality is characterised by behaviours that maximise their and/or their offspring’s personal safety, for example by recruiting allies who provide social and emotional support or physical protection, particularly in response to stressful circumstances (i.e. “tending and befriending”; Taylor et al. 2000). Indeed, recent experimental evidence suggests that facial cues to threat are more salient to men in contexts where violent male-male competition is likely to be intense (i.e. to maximise success in competition) but are more salient to women in contexts where self-protection is of greater concern (i.e. to avoid further threats; Watkins et al. 2013). Although prior research suggests that there are no sex differences in preferences for allies with a competitive advantage when the likelihood of winning is manipulated in economic games (Benenson et al. 2009), this does not rule out the possibility that preferences for facial masculinity-femininity in allies will vary differently for women versus men in light of recent experience of competition, especially as violent within-sex competition has lower benefits and greater costs for women more generally (reviewed in Archer 2009; Campbell 2013). Thus, we predict that recent experience (win or loss) of direct versus indirect competition will have different effects on how men judge allies (to facilitate successful competition) compared to how women judge allies (to seek emotional support and/or protection). While stronger preferences among men for dominant allies following losses in violent status contests (compared to wins) would function, in part, to recruit allies who are better placed to facilitate successful competition (i.e. parochial altruism; Choi and Bowles 2007; McDonald et al. 2012) and increase dominance rank (e.g. in primates; Schülke et al. 2010; Gilby et al. 2013; in humans; von Rueden et al. 2008, 2014), stronger preferences among women for prosocial allies following losses in violent contests for status (compared to wins) would function to recruit emotionally supportive (i.e. investing) partners (reviewed in Watkins et al. 2012a) and/or avoid dominance-related (i.e. low investing) characteristics in social partners when self-protection is at a premium. This prediction is particularly relevant to close female bonds (Taylor et al. 2000) if unsupportive close bonds are poor solutions to dealing with stressful circumstances (see Taylor 2006 for discussion) and proxies for these traits such as emotional coldness are gauged from masculine facial characteristics (Perrett et al. 1998). Moreover, this predicted pattern of findings is likely to be specific to when competition is direct (violent) as opposed to indirect (economic). For example as lower income relative to one’s peers negatively predicts measures of life satisfaction (Boyce et al. 2010) and as prior work suggests that attraction to facial femininity is greater when concerns about resource scarcity are salient (Little et al. 2007; Lee and Zietsch 2011), prosocial allies may be preferred when economic competition is intense such as when competing against peers for promotion, particularly in adverse circumstances where the costs of preferring dominance-related characteristics in social partners are greater, in light of, for example their low egalitarianism (e.g. Stirrat and Perrett 2010; Petersen et al. 2013). Thus, recent experience of economic competition may shape ally preference such that apparent dominance is avoided in allies (i.e. weaker preferences for facial masculinity) and/or prosocial individuals are sought as allies (i.e. stronger preferences for facial femininity).
Method
Participants
Two hundred forty-six participants (121 men, mean age = 22.75 years, SD = 6.03 years) completed the experiment online. Participants were recruited from links on social bookmarking sites, such as stumble upon. Previous research on social perceptions of faces has demonstrated that laboratory and online studies produce equivalent results (e.g. Watkins et al. 2010a, 2012b) and are comparable more generally (Gosling et al. 2004). Responses from duplicate IP addresses were not recorded. As the experiment was run online, recording of responses to the task is free from experimenter bias (i.e. as such, blinded methods were used when behavioural data were recorded).
Stimuli
Following previous studies of perceptions of masculinised versus feminised faces (e.g. Perrett et al. 1998; Jones et al. 2010; Watkins et al. 2010a, b), we used prototype-based image transformation to objectively and systematically manipulate sexually dimorphic aspects of 2D shape in digital face images. Following these studies, 50 % of the linear differences in 2D shape between symmetrised versions of a male and female prototype were added to or subtracted from digital face images of 20 young white adult men (M age = 19.5 years, SD = 2.3 years) and 20 young white adult women (M age = 18.4 years, SD = 0.7 years; see Tiddeman et al. 2001 for further technical details). The faces used here were of Canadian students and have been used in previous work on social perceptions of dominance (e.g. Watkins et al. 2010a, 2012b). The resultant masculinised and feminised versions of the individual faces images differ in sexually dimorphic aspects of 2D shape but are identical in other regards (e.g. identity, symmetry, skin colour and texture; Rowland and Perrett 1995). Examples of masculinised and feminised face images are shown in Fig. 1.
This process created 20 pairs of male face images and 20 pairs of female face images in total, with each pair consisting of a masculinised and feminised version of the same individual. These methods affect perceptions of dominance, physical strength and masculinity in the predicted manner (e.g. DeBruine et al. 2006; Jones et al. 2010). Masculinised versions of men’s and women’s faces are perceived as more dominant than feminised versions of men’s and women’s faces (e.g. Watkins et al. 2010a, 2012b).
Procedure
The experiment consisted of two phases: an initial priming phase and, subsequently, an ally preference test (adapted from Watkins and Jones 2012; Watkins et al. 2012a). In the initial priming phase of the experiment, each participant was randomly allocated to one of four conditions: a condition where they were instructed to imagine winning a physical fight, a condition where they were instructed to imagine losing a physical fight, a condition where they were instructed to imagine winning a contest for promotion and a condition where they were instructed to imagine losing a contest for promotion. Contests for promotion against same-sex colleagues have been used as scenarios in prior research on behavioural responses to competition (Griskevicius et al. 2009). The participants were given the following instructions: “Please take a moment to imagine that you have just been involved in a ‘physical fight’/‘contest for promotion’ with ‘someone’/‘a colleague’ of the same sex and age as you and that you ‘won’/‘lost’ the ‘fight’/‘contest’. Imagine how ‘winning the fight’/‘winning the contest’ made you feel/losing ‘the fight’/‘out on promotion’ made you feel and visualize yourself ‘winning’/‘losing’ ‘the fight’/‘being told that you were favoured over your colleague’/‘your colleague was favoured over you’”. Participants were allocated to one experimental scenario only. Before moving on to the ally preference test, participants rated how vividly they had imagined the scenario on a 1 (not very vivid) to 7 (very vivid) scale. Participants can accurately rate the vividness of their mental imagery (Pearson et al. 2011).
Immediately after the priming phase of the experiment, the participants completed an ally preference test. In this test, the participants were shown 20 pairs of male faces and 20 pairs of female faces, with each pair consisting of a masculinised and feminised version of the same individual. On each trial, the participants were asked to indicate which face in each pair they thought would make the better ally, and how much more suitable that individual would be as an ally. The participants were asked to choose the better ally and indicate their preference for the chosen face (relative to the other face in the pair) using the response options “much better ally”, “better ally”, “somewhat better ally” and “slightly better ally”. Trial order, the sex of face and the side of the screen on which any given image (masculinised or feminised) was shown were all fully randomised.
Initial processing of data
Following prior work on systematic variation in judgements of masculinised versus feminised versions of faces (e.g. Watkins et al. 2010b), responses were coded using the following scale:
-
1.
0–3: feminised face rated a much better ally (=0), better ally (=1), somewhat better ally (=2) or slightly better ally (=3) than the masculinised face.
-
2.
4–7: masculinised face rated a slightly better ally (=4), somewhat better ally (=5), better ally (=6) or much better ally (=7) than the feminised face.
We used this data to calculate a participant’s average score on the ally preference test, separately for judgements of men’s faces and judgements of women’s faces. Higher values indicate a stronger tendency to perceive masculine individuals as better allies than feminine individuals. Use of np2 in analyses indicates the effect size measure partial eta squared.
Results
First we carried out initial one-sample t tests to compare overall preferences for masculinity-femininity in allies with what would be expected by chance alone (i.e. 3.5). These analyses revealed that while participants tended to perceive masculinised versions of men’s faces to be better allies than feminised versions of men’s faces (M = 3.61, 95 % CI [−0.00, 0.23]; t(245) = 1.94; p = 0.054, r = 0.12), they perceived feminised versions of women’s faces to be better allies than masculinised versions of women’s faces (M = 3.22, 95 % CI [−0.38, −0.17]; t(245) = 5.20; p < 0.001, r = 0.32). Analysing the data separately for male and female judges revealed that men preferred masculine men more than feminine men as allies (M = 3.73, 95 % CI [0.06, 0.40]; t(121) = 2.64; p < 0.01, r = 0.23), but women had no overall preference for masculine or feminine men as allies (M = 3.50, 95 % CI [−0.15, 0.16]; t(125) = 0.01; p = 0.99). Both men (M = 3.24, 95 % CI [−0.42, −0.11]; t(121) = 3.33; p < 0.01, r = 0.29) and women (M = 3.21, 95 % CI [−0.43, −0.15]; t(125) = 4.04; p < 0.01, r = 0.34) preferred feminine women more than masculine women as allies.
Next, we carried out a mixed design ANOVA with scores on the ally preference test as the dependent variable; the within-subject factor was face sex (men’s faces, women’s faces) and the between subjects factors were contest type (physical fight, promotion), contest outcome (win, loss) and sex of participant (male, female). These analyses revealed a significant main effect of face sex (F(1, 238) = 65.17; p < 0.001, np2 = 0.22), whereby participants were more likely to choose masculine allies when judging men’s faces (M = 3.61, SEM = 0.06) than women’s faces (M = 3.22, SEM = 0.05). This main effect was qualified by an interaction with contest type (F(1, 238) = 6.78; p = 0.01, np2 = 0.03), however. This interaction indicated that the tendency to prefer masculinity more in allies when judging men than when judging women was significantly greater when judging allies after physical fights (M male = 3.96, SEM = 0.09, M female = 3.44, SEM = 0.08, r = 0.34) than when judging allies after contests for promotion (M male = 3.25, SEM = 0.06, M female = 3.00, SEM = 0.07, r = 0.14). We also observed a significant interaction between face sex and sex of participant (F(1, 238) = 6.04; p = 0.015, np2 = 0.03). This interaction demonstrated that the tendency to prefer masculine allies more when judging men than women was greater in male participants (r = 0.27) than it was in female participants (r = 0.21).
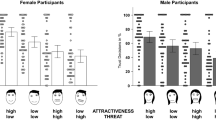
We also observed a significant effect of contest type (F(1, 238) = 39.27; p < 0.001, np2 = 0.14). This effect reflected a stronger preference for masculine allies following a physical fight (M = 3.70, SEM = 0.08) than following a contest for promotion (M = 3.12, SEM = 0.05). A significant interaction between contest outcome and participant sex (F(1, 238) = 4.22; p < 0.05, np2 = 0.02) indicated that men’s preference for masculine allies was stronger after a loss than a win in a contest for status while women’s preference for masculine allies was weaker after a loss than a win in a contest for status (both ts < 1.60, both ps > 0.11). Of central interest to our hypotheses, we observed a significant three-way interaction between contest type, contest outcome and participant sex (F(1, 238) = 16.14; p < 0.001, np2 = 0.06; see Fig. 2). No other significant effects or interactions were found (all Fs < 3.13, all ps > 0.07).
The significant interaction between contest type, contest outcome and participant sex on preferences for facial cues related to dominance in allies (p < 0.001, np2 = 0.06). While men’s preference for dominance-related characteristics in allies are stronger after a loss compared to a win in a violent contest for status (r = 0.42; a), women’s preference for dominance-related characteristics in allies are weaker after a loss compared to a win in a violent contest for status (r = 0.25; b). Error bars show 95 % confidence intervals
In order to interpret our significant three-way interaction between contest type, contest outcome and participant sex, we conducted separate ANOVAs for men and women, collapsed across sex of face. This analysis revealed that contest type (F(1, 117) = 29.98; p < 0.01, np2 = 0.20) and contest outcome (F(1, 117) = 4.90; p = 0.03, np2 = 0.04), as well as an interaction between contest type and contest outcome (F(1, 117) = 9.12; p < 0.01, np2 = 0.07) had significant effects on men’s preference for facial cues to dominance in allies. Independent samples t tests revealed that while men’s preferences for facial cues to dominance in allies was stronger following a loss (M = 4.16, SEM = 0.16) compared to a win (M = 3.51, SEM = 0.11) in a physical fight (t(52.63) = 3.38, p < 0.01, r = 0.42, 95 % CI [0.27, 1.05]), they did not alter according to the outcomes of a contest for promotion (t(59) = 0.64, p = 0.53, r = 0.08, 95 % CI [−0.42, 0.22]).
When analysing women’s data, there was a significant effect of contest type (F(1, 121) = 11.70; p < 0.01, np2 = 0.09) but not contest outcome (F(1, 121) = 0.51; p = 0.48) on their preference for facial cues to dominance in allies. The interaction between contest type and contest outcome was significant, however (F(1, 121) = 7.12; p < 0.01, np2 = 0.06). Women’s preferences for facial cues to dominance in allies were weaker following a loss (M = 3.34, SEM = 0.14) compared to a win (M = 3.78, SEM = 0.15) in a physical fight (t(65) = 2.12; p = 0.04, r = .25, 95 % CI [−0.85, −0.03]) but tended to be stronger following a loss (M = 3.24, SEM = 0.10) compared to a win (M = 2.99, SEM = 0.10) in a contest for promotion (t(56) = 1.78; p = 0.08, r = 0.23, 95 % CI [−0.03, 0.54]).
Follow-up one-sample t tests against chance (i.e. 3.5) for each of our experimental conditions (separated by sex of participant) demonstrated that men preferred masculine allies after a loss in a physical fight (M = 4.16, 95 % CI [0.34, 0.99]; t(29) = 4.19; p < 0.001, r = 0.36) and preferred feminine allies after both a loss (M = 3.10, 95 % CI [−0.60, −0.21]; t(34) = 4.15; p < 0.001, r = 0.33) and win in a contest for promotion (M = 3.20, 95 % CI [−0.57, −0.04]; t(25) = 2.33; p = 0.03, r = 0.22). Women preferred feminine allies after both a loss (M = 3.24, 95 % CI [−0.47, −0.05]; t(25) = 2.52; p = 0.02, r = 0.24) and a win in a contest for promotion (M = 2.99, 95 % CI [−0.71, −0.31]; t(31) = 5.21; p < 0.001, r = 0.42). In our remaining priming conditions, men or women did not prefer masculine-feminine allies at levels that differed from chance (all ts < 1.91, all ps > 0.07). Of note, even where preferences for masculine-feminine allies do not differ from chance, and of central interest to our hypotheses, our higher order three-way interaction demonstrates that men’s social responses to allies differ significantly from women’s social responses to allies. Moreover, our separate ANOVAs for men and women demonstrate relative strengthening/weakening of preferences for facial masculinity-femininity in allies according to our primed experimental contexts.
A separate ANOVA on the dependent variable rated vividness of imagery, with the between-subject factors contest type, contest outcome and participant sex, confirmed that the rated vividness of imagery was equivalent across our four scenarios and that men and women did not differ from one another in their rated vividness of mental imagery across these scenarios (all Fs < 1.97, all ps > 0.16). Rerunning all analyses with participant age entered as a covariate in the model did not alter any of our results.
Discussion
Here we report novel evidence that sexually dimorphic characteristics are used to assess individuals as potential allies. Firstly, both men and women, on average and across contexts, associated feminine shape cues in other women with their suitability as an ally. In contrast, and consistent with our initial predictions, while men associated masculine shape cues in other men with suitability as an ally, women had no overall preference for masculine or feminine men as allies. As masculine (i.e. dominant looking; Puts 2010) men are better-placed to provide leverage as allies via the threat they pose to external groups (i.e. parochial altruism; Choi and Bowles 2007; McDonald et al. 2012; von Rueden et al. 2014), these initial findings are consistent with our prediction that men’s preference for masculine allies functions, in part, to improve dominance rank. Preference for dominant-looking (i.e. masculine) allies may, in turn, facilitate successful competition against rival groups, an important concern for males over evolutionary history (Bowles 2009; Benenson et al. 2013; see also van Vugt and Grabo 2015 for discussion of dominance and leadership in general contexts).
Additionally, our priming experiment suggests facultative responses to facial cues related to dominance in light of the (i) means and (ii) outcomes of recent status contests. Our significant three-way interaction suggests sex-specific preferences for cues to dominance (i.e. masculine faces) in light of recent experience of intrasexual competition. While men’s preference for dominance-related characteristics in allies was stronger following losses (compared to wins) in violent contests for status, women’s preference for dominance-related characteristics in allies was weaker following losses (compared to wins) in violent contests for status. These findings are consistent with the proposal that while men would choose allies in light of recent losses in order to provide leverage and facilitate successful competition (e.g. Schülke et al. 2010), women would choose allies in light of recent losses in order to seek prosocial/supportive allies (Watkins et al. 2012a) and/or to avoid formidable allies following aggressive conflict, where the costs to pursuing such relationships may be particularly substantial. While research has explored context-dependent judgements of cues related to dominance in romantic (reviewed in Little et al. 2011) and competitive interactions (see Fessler and Holbrook 2013 for recent discussion), this work provides novel evidence for contextual factors that shape associate choice. The facultative responses reported here may play an important role in human sociality when confronted by new environments or groups.
Although our higher-order interaction demonstrates that the outcomes of different forms of status competition have different effects on men’s versus women’s preferences for traits related to dominance in allies, post hoc tests suggest that the effects of recent experience of economic competition (competing for promotion) on preferences for traits related to dominance in allies are less clear. These tests suggest that women tend to strengthen their preference for dominance-related characteristics in allies after failing in a contest for promotion against a same-sex peer (compared to succeeding). While the effects of our “minimal manipulation” may well be substantial when tested in the field (Prentice and Miller 1992), future work could test for effects of recent experience on ally choice in organisations or groups, using complementary methods such as behavioural observation or experimental war games (see Johnson et al. 2006). It is worth noting however that our experimental manipulation suggests that minimal cues are sufficient to moderate ally choice, demonstrating great flexibility in the cognitive architecture that underpins sociality in humans.
Potential limitations of our work require further discussion. For example in non-humans, coalitions, where two conspecifics simultaneously aggress against a third party, differ from alliances, where coalitions are revisited and reformed over time, usually against multiple opponents (Harcourt and de Waal 1992). Here, our participants were asked to judge others for their suitability as potential allies from face cues alone. While our data do not speak directly to the time course of this phenomena in humans (i.e. short-term stable alliances versus longer-term repeated alliances), our findings demonstrate that short-term changes to the nature of the environment alter social judgements of allies. Further work on responses to allies in the short- versus long-term would likely prove fruitful, particularly in light of the multi-level complexity of human alliance politics over time and space (Snyder 2007) and the functional basis of episodic memory in humans for simulating future outcomes based on past transactions (see Suddendorf and Corballis 2007 for discussion). Secondly, although the results from our minimal manipulation, whereby we simply ask people to imagine themselves in different scenarios, suggest that similar effects in the real world may be substantial (Prentice and Miller 1992), further work could potentially test for behavioural responses to potential allies following actual contest outcomes, for example among professional fighters, sportspeople or work colleagues. Finally, paradigms other than the forced choice paradigm used here may also be fruitful for investigating the decision-making processes involved in ally choice, such as observation of same-sex group behaviour within the laboratory or field.
In summary, we used experimental priming techniques to demonstrate that competition-related factors have direct effects on the “trade-off” between preferring dominant versus prosocial characteristics in allies and that these effects differ for men’s versus women’s judgements of allies in ways that can be understood in light of research on sex differences in primate sociality (e.g. Bowles 2009; Benenson et al. 2013). Theoretical perspectives suggest that an important factor in the evolution of the social brain, or “Machiavellian intelligence” (see Dunbar and Shultz 2007) was the increasing threat posed by competition for scarce resources as humans began to master their surrounding environment (Flinn et al. 2005). Our findings provide direct experimental evidence that intrasexual competition may have been an important factor in shaping the cognitive architecture that underpins alliance formation, a key facet of social intelligence in humans.
References
Ackerman JM, Shapiro JR, Neuberg SL, Kenrick DT, Becker DV, Griskevicius V, Maner JK, Schaller M (2006) They all look the same to me (unless they’re angry): from out-group homogeneity to out-group heterogeneity. Psychol Sci 17:836–840
Archer J (2009) Does sexual selection explain human sex differences in aggression? Behav Brain Sci 32:249–311
Benenson JF, Markovits H, Emery Thompson M, Wrangham RW (2009) Strength determines coalitional strategies in humans. Proc R Soc Lond B 276:2589–2595
Benenson JF, Markovits H, Hultgren B, Nguyen T, Bullock G, Wrangham R (2013) Social exclusion: more important to human females than males. PLoS One 8:e55851
Bergeron P, Grignolio S, Apollonio M, Shipley B, Festa-Bianchet M (2010) Secondary sexual characters signal fighting ability and determine social rank in Alpine ibex (Capra ibex). Behav Ecol Sociobiol 64:1299–1307
Bowles S (2009) Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324:1293–1298
Boyce CJ, Brown GDA, Moore SC (2010) Money and happiness: rank of income, not income, affects life satisfaction. Psychol Sci 21:471–475
Campbell A (2013) The evolutionary psychology of women’s aggression. Philos T Roy Soc B 368:20130078
Caro TM, Collins DA (1987) Male cheetah social organization and territoriality. Ethology 74:52–64
Choi JK, Bowles S (2007) The coevolution of parochial altruism and war. Science 318:636–640
Connor RC, Heithaus MR, Barre LM (1999) Superalliance of bottlenose dolphins. Nature 371:571–572
Connor RC, Heithaus MR, Barre LM (2001) Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance’. Proc R Soc Lond B 268:263–267
DeBruine LM, Jones BC, Little AC, Boothroyd LG, Perrett DI, Penton-Voak IS, Cooper PA, Penke L, Feinberg DR, Tiddeman BP (2006) Correlated preferences for facial masculinity and ideal or actual partner’s masculinity. Proc R Soc Lond B 273:1355–1360
DeScioli P, Kurzban R (2009) The alliance hypothesis for human friendship. PLoS One 4:e5802
Duffy KG, Wrangham RW, Silk JB (2007) Male chimpanzees exchange political support for mating opportunities. Curr Biol 17:R586–R587
Dunbar RIM, Shultz S (2007) Evolution in the social brain. Science 317:1344–1347
Emlen DJ (2008) The evolution of animal weapons. Annu Rev Ecol Evol S 39:387–413
Feh C (1999) Alliances and reproductive success in Camargue stallions. Anim Behav 57:705–713
Fessler DMT, Holbrook C (2013) Friends shrink foes: the presence of comrades decreases the envisioned physical formidability of an opponent. Psychol Sci 24:797–802
Flinn MV, Geary DC, Ward CV (2005) Ecological dominance, social competition, and coalitionary arms races: why humans evolved extraordinary intelligence. Evol Hum Behav 26:10–46
Fraser ON, Bugnyar T (2012) Reciprocity of agonistic support in ravens. Anim Behav 83:171–177
Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE (2013) Fitness benefits of coalitionary aggression in male chimpanzees. Behav Ecol Sociobiol 67:373–381
Gosling SD, Vazire S, Srivastava S, John OP (2004) Should we trust web-based studies? A comparative analysis of six preconceptions about internet questionnaires. Am Psychol 59:93–104
Griskevicius V, Tybur JM, Gangestad SW, Perea EF, Shapiro JR, Kenrick DT (2009) Aggress to impress: hostility as an evolved context-dependent strategy. J Person Soc Psychol 96:980–994
Hand JL (1986) Resolution of social conflicts: dominance, egalitarianism, spheres of dominance, and game theory. Q Rev Biol 61:201–220
Harcourt AH, de Waal FBM (1992) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford
Henrich J, Gil-White F (2001) The evolution of prestige: freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol Hum Behav 22:165–196
Hill RA, Dunbar RIM (2003) Social network size in humans. Hum Nat 14:53–72
Holt-Lunstad J, Smith TB, Layton JB (2010) Social relationships and mortality risk: a meta-analytic review. PLoS Med 7:e1000316
Hsu Y, Earley RL, Wolf LL (2006) Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev 81:33–74
Johnson DDP, McDermott R, Barrett ES, Cowden J, Wrangham R, McIntyre MH, Rosen SP (2006) Overconfidence in wargames: experimental evidence on expectations, aggression, gender and testosterone. Proc R Soc Lond B 273:2513–2520
Jones BC, DeBruine LM, Main JC, Little AC, Welling LLM, Feinberg DR, Tiddeman BP (2010) Facial cues of dominance modulate the short-term gaze-cuing effect in human observers. Proc R Soc Lond B 277:617–624
Lawson DW, Mace R (2011) Parental investment and the optimization of human family size. Philos T Roy Soc B 366:333–343
Lee AJ, Zietsch BP (2011) Experimental evidence that women’s mate preferences are directly influenced by cues of pathogen prevalence and resource scarcity. Biol Lett 7:892–895
Little AC, Cohen DL, Jones BC, Belsky J (2007) Human preferences for facial masculinity change with relationship type and environmental harshness. Behav Ecol Sociobiol 61:967–973
Little AC, Jones BC, DeBruine LM (2011) Facial attractiveness: evolutionary based research. Philos T Roy Soc Lond B 366:1638–1659
Macfarlan SJ, Walker RS, Flinn MV, Chagnon NA (2014) Lethal coalitionary aggression and long-term alliance formation among Yanomamö men. P Natl Acad Sci USA 111:16662–16669
Malo AF, Roldan ERS, Garde JJ, Soler AJ, Vicente J, Gortazar C, Gomendio M (2009) What does testosterone do for red deer males? Proc R Soc Lond B 276:971–980
McDonald MM, Navarrete CD, Van Vugt M (2012) Evolution and the psychology of intergroup conflict: the male warrior hypothesis. Philos T Roy Soc Lond B 367:670–679
Noë R, Sluijter A (1995) Which adult male savanna baboons form coalitions? Int J Primatol 16:77–105
Packer C, Pusey AE (1982) Cooperation and competition within coalitions of male lions: kin selection or game theory? Nature 296:740–742
Patzelt A, Kopp GH, Ndao I, Kalbitzer U, Zinner D, Fischer J (2014) Male tolerance and male-male bonds in a multilevel primate society. P Natl Acad Sci USA 111:14740–14745
Pearson J, Rademaker R, Tong F (2011) Evaluating the mind’s eye: the metacognition of visual imagery. Psychol Sci 22:1535–1542
Pelletier F, Festa-Bianchet M (2006) Sexual selection and social rank in bighorn rams. Anim Behav 71:649–655
Perrett DI, Lee KJ, Penton-Voak IS, Rowland DR, Yoshikawa S, Burt DM, Henzi SP, Castles DI, Akamatsu S (1998) Effects of sexual dimorphism on facial attractiveness. Nature 394:884–887
Perry S, Barrett HC, Manson JH (2004) White-faced capuchin monkeys show triadic awareness in their choice of allies. Anim Behav 67:165–170
Petersen MB, Sznycer D, Sell A, Cosmides L, Tooby J (2013) The ancestral logic of politics: upper body strength regulates men’s assertion of self-interest over economic redistribution. Psychol Sci 24:1098–1103
Prentice DA, Miller DT (1992) When small effects are impressive. Psychol Bull 112:160–164
Preston BT, Stevenson IR, Pemberton JM, Coltman DW, Wilson K (2003) Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc R Soc Lond B 270:633–640
Puts DA (2010) Beauty and the beast: mechanisms of sexual selection in humans. Evol Hum Behav 31:157–175
Queller DC (2011) Expanded social fitness and Hamilton’s rule for kin, kith, and kind. P Natl Acad Sci USA 108:10792–10799
Rowland DA, Perrett DI (1995) Manipulating facial appearance through shape and colour. IEEE Comput Graph 15:70–76
Santos ESA, Scheck D, Nakagawa S (2011) Dominance and plumage traits: meta-analysis and metaregression analysis. Anim Behav 82:3–19
Schülke O, Bhagavatula J, Vigilant L, Ostner J (2010) Social bonds enhance reproductive success in male macaques. Curr Biol 20:2207–2210
Silk JB, Alberts SC, Altmann J (2004) Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim Behav 67:573–582
Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL (2010) Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol 20:1359–1361
Smallegange IM, van der Meer J, Kurvers RHJM (2006) Disentangling interference competition from exploitative competition in a crab-bivalve system using a novel experimental approach. Oikos 113:157–167
Snyder GH (2007) Alliance politics. Cornell University Press, Ithaca, NY
Stirrat M, Perrett DI (2010) Valid facial cues to cooperation and trust: male facial width and trustworthiness. Psychol Sci 21:349–354
Suddendorf T, Corballis MC (2007) The evolution of foresight: what is mental time travel, and is it unique to humans? Behav Brain Sci 30:299–313
Taylor SE (2006) Tend and befriend: biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci 15:273–277
Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA (2000) Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev 107:411–429
Tiddeman BP, Burt DM, Perrett DI (2001) Prototyping and transforming facial textures for perception research. IEEE Comput Graph 21:42–50
Todorov A, Said CP, Engell AD, Oosterhof NN (2008) Understanding evaluation of faces on social dimensions. Trends Cogn Sci 12:455–460
Van Vugt M, Grabo AE (2015) The many faces of leadership: an evolutionary-psychology approach. Curr Dir Psychol Sci 24:484–489
von Rueden C, Gurven M, Kaplan H (2008) The multiple dimensions of male social status in an Amazonian society. Evol Hum Behav 29:402–415
von Rueden C, Gurven M, Kaplan H (2011) Why do men seek status? Fitness payoffs to dominance and prestige. Proc R Soc Lond B 278:2223–2232
von Rueden C, Gurven M, Kaplan H, Stieglitz J (2014) Leadership in an egalitarian society. Hum Nat 25:538–566
Watkins CD, Jones BC (2012) Priming men with different contest outcomes modulates their dominance perceptions. Behav Ecol 23:539–543
Watkins CD, Fraccaro PJ, Smith FG, Vukovic J, Feinberg DR, DeBruine LM, Jones BC (2010a) Taller men are less sensitive to cues of dominance in other men. Behav Ecol 21:943–947
Watkins CD, Jones BC, DeBruine LM (2010b) Individual differences in dominance perception: dominant men are less sensitive to facial cues of male dominance. Pers Indiv Differ 49:967–971
Watkins CD, DeBruine LM, Little AC, Jones BC (2012a) Social support influences preferences for feminine facial cues in potential social partners. Exp Psychol 59:340–347
Watkins CD, Quist M, Smith FG, DeBruine LM, Jones BC (2012b) Individual differences in women’s perceptions of other women’s dominance. Eur J Personality 26:79–86
Watkins CD, DeBruine LM, Feinberg DR, Jones BC (2013) A sex difference in the context-sensitivity of dominance perceptions. Evol Hum Behav 34:366–372
Welling LLM, Moreau BJP, Bird BM, Hansen S, Carre JM (2016) Exogenous testosterone increases men’s perceptions of their own physical dominance. Psychoneuroendocrino 64:136–142
Whitehead H, Connor RC (2005) Alliances I: how large should alliances be? Anim Behav 69:117–126
Acknowledgments
The authors wish to thank the editor and reviewers for their helpful comments on our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were in accordance with the ethical standards of our institutions’ ethics committees.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All participants provided informed consent.
Funding
BCJ is funded by a European Research Council Starting Grant 282655 (OCMATE).
Additional information
Communicated by R. Noë
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Watkins, C.D., Jones, B.C. Competition-related factors directly influence preferences for facial cues of dominance in allies. Behav Ecol Sociobiol 70, 2071–2079 (2016). https://doi.org/10.1007/s00265-016-2211-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2211-2