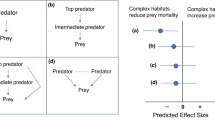
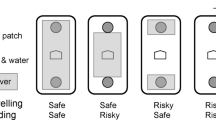
Abstract
Previous studies indicate that when predation risk is uniform across habitats, foragers concentrate their exploitation in fewer patches. Although uniform predation risk may seem rare in nature, some scenarios might cause it. Testing all scenarios in a single experiment is unfeasible; therefore, we developed a model that points whether concentration of exploitation in specific habitats due to uniform risk requires parameter values similar to what is found in literature. This model was based on Brown’s (Behav Ecol Sociobiol 22:37–47, 1988) fitness function but rescaled to multiple habitats and predators, including uniform risk predators. Deriving function’s maximum allowed comparisons with giving-up density studies. Results showed that uniform predation risk had a u-shaped effect in habitat exploitation, causing a concentration of habitat exploitation at probabilities of survival from 0.2 to 0.8. However, the length of this interval and degree of concentration depended on the value of safety to forager fitness. Heterogeneous, nonuniform, predation risk decreases habitat exploitation where it was higher, therefore suppressing the effect of uniform risk on prey behavior. Time spent in the focal habitat and metabolic costs reduced the detectability of habitat concentration, while total time did not. We also found that uniform risk reduced accuracy of heterogeneous risk measurements. Future studies should aim to control all possible predators, as even the mild ones can induce complex behavior.





Similar content being viewed by others
References
Abu Baker MA, Brown JS (2010) Islands of fear: effects of wooded patches on habitat suitability of the striped mouse in a South African grassland. Funct Ecol 24:1313–1322
Abu Baker MA, Brown JS (2011) Patch use behaviour of Elephantulus myurus and Micaelamys namaquensis: the role of diet, foraging substrates and escape substrates. Afr J Ecol 50:167–175
Bouskila A (2012) Interactions between predation risk and competition: a field study of kangaroo rats and snakes. Ecology 76:165–178
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Brown JS (1992) Patch use under predation risk: I. models and predictions. Ann Zool Fenn 29:301–309
Brown JS, Arel Y, Abramsky Z, Kotler BP (1992a) Patch use by gerbils (Gerbillus allenbyi) in sandy and rocky habitats. J Mammal 73:821–829
Brown JS, Morgan RA, Dow BD (1992b) Patch use under predation risk: II. a test with fox squirrels, Sciurus niger. Ann Zool Fenn 29:311–318
Brown JS, Kotler BP, Valone TJ (1994) Foraging under predation: a comparison of energetic and predation costs in a Negev and Sonoran desert rodent community. Aust J Zool 42:435–438
Brown JS, Kotler BP, Mitchell W (1997) Competition between birds and mammals: a comparison of giving-up densities between crested larks and gerbils. Evol Ecol 11:757–771
Brown JS, Laundré JW, Gurung M (1999) The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80:385–399
Brown JS, Kotler BP, Bouskila A (2001) Ecology of fear: foraging games between predators and prey with pulsed resources. Ann Zool Fenn 38:71–87
Charnov EL (1976) Optimal foraging: the marginal value theorem. Theor Popul Biol 9:129–136
Dickman CR, Greenville AC, Beh C, Tamayo B, Wardle M (2010) Social organization and movements of desert rodents during population “booms” and “busts” in central Australia. J Mammal 91:798–810
Druce D, Brown JS, Castley JG, Kerley GIH, Kotler BP, Slotow RH, Knight MH (2006) Scale dependent foraging costs: habitat use by rock hyraxes (Procavia capensis) determined using giving-up densities. Oikos 3:513–525
Druce DJ, Brown JS, Kerley GIH, Kotler BP, Mackey RL, Slotow R (2009) Spatial and temporal scaling in habitat utilization by klipspringers (Oreotragus oreotragus) determined using giving-up densities. Austral Ecol 34:577–587
Eccard JA, Liesenjohann T (2008) Foraging decisions in risk-uniform landscapes. PLoS One 3:e3438
Eccard JA, Pusenius J, Sundell J, Halle S, Ylönen H (2008) Foraging patterns of voles at heterogeneous avian and uniform mustelid predation risk. Oecologia 157:725–34
Fanson BG, Fanson KV, Brown JS (2008) Foraging behaviour of two rodent species inhabiting a kopje (rocky outcrop) in Tsavo West National Park, Kenya. Afr Zool 43:184–191
Gilliam J, Fraser D (1987) Habitat selection under predation hazard: test of a model with foraging minnows. Ecology 68:1856–1862
Hamilton WD (1971) Geometry for the selfish herd. J Theor Biol 31:295–311
Jacob J, Brown JS (2000) Microhabitat use, giving-up densities and temporal activity as short- and long-term anti-predator behaviors in common voles. Oikos 91:131–138
Korpimäki E, Koivunen V, Hakkarainen H (1996) Microhabitat use and behavior of voles under weasel and raptor predation risk: predator facilitation? Behav Ecol 7:30–34
Kotler BP, Blaustein L (1995) Titrating food and safety in a heterogeneous environment: when are the risky and safe patches of equal value? Oikos 74:251–258
Kotler BP, Brown JS, Slotow RH, Goodfriend WL, Strauss M (1993) The influence of snakes on the foraging behavior of gerbils. Oikos 67:309–316
Kotler BP, Ayal Y, Subach A (1994) Effects of predatory risk and resource renewal on the timing of foraging activity in a gerbil community. Oecologia 100:391–396
Laundré JW, Hernández L, Altendorf KB (2001) Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, U.S.A. Can J Zool 79:1401–1409
Liesenjohann T, Eccard JA (2008) Foraging under uniform risk from different types of predators. BMC Ecol 8:19
Lima SL (1987) Vigilance while feeding and its relation to the risk of predation. J Theor Biol 124:303–316
Lima SL (1988) Initiation and termination of daily feeding in dark-eyed juncos: influences of predation risk and energy reserves. Oikos 53:3–11
Lima SL (1992) Life in a multi-predator environment: some considerations for anti-predatory vigilance. Ann Zool Fenn 29:217–226
Lima SL, Bednekoff PA (2008) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Dill LM (1990) Behavioral decisions made under risk of predation: a review and prospectus. Can J Zool 68:619–640
Mandelik Y, Jones M, Dayan T (2003) Structurally complex habitat and sensory adaptations mediate the behavioural responses of a desert rodent to an indirect cue for increased predation risk. Evol Ecol Res 5:501–515
Olsson O, Brown JS, Smith HG (2002) Long- and short-term state-dependent foraging under predation risk: an indication of habitat quality. Anim Behav 63:981–989
Orrock JL, Danielson BJ (2004) Rodents balancing a variety of risks: invasive fire ants and indirect and direct indicators of predation risk. Oecologia 140:662–7
Oyugi JO, Brown JS (2003) Giving-up densities and habitat preferences of European starlings and American robins. Condor 105:130
Pitt WC (1999) Effects of multiple vertebrate predators on grasshopper habitat selection: trade-offs due to predation risk, foraging, and thermoregulation. Evol Ecol 13:499–515
Price MV, Waser NM, Bass TA (1984) Effects of moonlight on microhabitat use by desert rodents. J Mammal 65:353–356
Rieucau G, Vickery WL, Doucet GJ (2009) A patch use model to separate effects of foraging costs on giving-up densities: an experiment with white-tailed deer (Odocoileus virginianus). Behav Ecol Sociobiol 63:891–897
Rosenheim JA (2004) Top predators constrain the habitat selection games played by intermediate predators and their prey. Isr J Zool 50:129–138
Royston P, Altman DG, Sauerbrei W (2006) Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 25:127–41
Shapira I, Sultan H, Shanas U (2008) Agricultural farming alters predator–prey interactions in nearby natural habitats. Anim Conserv 11:1–8
Shrader AM, Kerley GIH, Brown JS, Kotler BP (2012) Patch use in free-ranging goats: does a large mammalian herbivore forage like other central place foragers? Ethology 118:967–974
Sih A (1998) Game theory and predator-prey response races. In: Dugatin LA, Reeve H (ed) Game theory and animal behavior, 1st edn. Oxford, pp 221–238
Stapp P, Lindquist MD (2007) Roadside foraging by kangaroo rats in a grazed short-grass prairie landscape. West North Am Nat 67:368–377
Stephens DW, Brown JS, Ydenberg RC (2007) Foraging behavior and ecology. The University of Chicago Press, Chicago and London
Tsurim I, Kotler BP, Gilad A, Elazary S, Abramsky Z (2010) Foraging behavior of an urban bird species: molt gaps, distance to shelter, and predation risk. Ecology 91:233–41
Vapnyarskii IB (1994) Lagrange multipliers. Encyclopedia of Mathematics. http://www.encyclopediaofmath.org/index.php?title=Lagrange_multipliers&oldid=32304. Accessed 1st August 2014
Verdolin JL (2006) Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464
Vickery WL, Rieucau G, Jean Doucet G (2011) Comparing habitat quality within and between environments using giving up densities: an example based on the winter habitat of white-tailed deer Odocoileus virginianus. Oikos 120:999–1004
Wasserberg G, Abramsky Z, Valdivia N, Kotler BP (2005) The role of vegetation characteristics and foraging substrate in organizing a centrifugal gerbil community. J Mammal 86:1009–1014
Acknowledgments
We thank Bernardo Araujo, Mauricio Silveira, and the thesis committee for comments on the manuscript and on the general numeric exploration; this work was supported by the Brazilian Enterprise for Agricultural Research (EMBRAPA—PANTANAL) and by a National Counsel of Technological and Scientific Development (CNPq) scholarship. We are also grateful for the comments of two anonymous reviewers about this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Lindström
Appendix
Appendix
Mathematical demonstration that uniform predation risk is proportional to variance in habitat use, considering this study model
This section is a supplementary electronic material of the following: Menezes, JFS; Kotler, BP.; and Mourão, GM. Uniform predation risk in nature: common, inconspicuous, and a source of error to predation risk experiments published at behavioral ecology and sociobiology. Corresponding author, Menezes, Jorge F.S., is affiliated to Empresa Brasileira de Pecuária e Agricultura—Rua 21 de Setembro, 1880 - Bairro Nossa Senhora de Fátima. CP 109 - Corumbá, MS- Brazil – ZIP: 79320-900. Email: jorgefernandosaraiva@gmail.com.
A relationship between variance in habitat use and uniform risk can be proven by stating that missed opportunity costs are proportional to mean resource in patches after controlling the effects of predation risk and alternate activities. This relation was reported in the beginning of foraging ecology (Charnov 1976) and has been used in several modern methods (Rieucau et al. 2009). In our model, opportunity costs are represented by \( \frac{\left(i-1\right)\phi }{p_i{p}_u\left(\partial F/\partial e\right)} \), where the denominator accounts for the effect of predation risk, in the missed opportunity cost. It follows that the numerator equals the effect of mean resource patches and the effect of alternate activities. The most parsimonious way to model both effect is as follows:
where \( \overline{f} \) is the average harvest rate of all habitats j ≠ i, representing mean resource in patches; b is an unknown constant representing the influence of resources in missing opportunity costs; and d represents alternative activities that contribute to missing opportunity costs (such as grooming and sleeping).
Substituting ϕ i in Formula 5:
This equation can be rearranged to show the relation between f i and \( \overline{f} \):
There are no square terms in the right side of this equation. Therefore, it is proportional to f i − \( \overline{f} \). Given that condition, uniform risk is inversely proportional to f i − \( \overline{f} \), and consequently, directly proportional to (f i − \( \overline{f} \))2, which is the numerator of the variance formula.
Rights and permissions
About this article
Cite this article
Menezes, J.F.S., Kotler, B.P. & Mourão, G.M. Uniform predation risk in nature: common, inconspicuous, and a source of error to predation risk experiments. Behav Ecol Sociobiol 68, 1809–1818 (2014). https://doi.org/10.1007/s00265-014-1790-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1790-z