Abstract
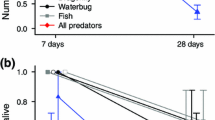
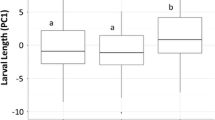
Hatching, the life history switch point between embryonic and larval or subadult stages, has traditionally been regarded as a fixed event in an organism’s development. This notion has been challenged by reports of environmentally cued hatching in recent years, which show embryos improve fitness by hatching in response to mortality risks. Here, we present evidence of accelerated hatching due to predation at two points during embryonic development in Chiromantis hansenae. Young embryos (0 day old) exposed to simulated predation hatched earlier compared to undisturbed clutches. Old embryos (4 days old) subjected to direct katydid predation had more immediate responses, hatching <1 h after predation on average. Hatching time was not correlated with female frog size, egg attendance time, or other predator cues. Results confirm predator-cued hatching in a new family of amphibians and support hatching plasticity being a widespread and potentially ancestral condition. We suggest mechanisms and ecological basis of cue transmission and response in C. hansenae and point out potential further research.



Similar content being viewed by others
References
Armstrong AF, Blackburn HN, Allen JD (2013) A novel report of hatching plasticity in the phylum Echinodermata. Am Nat 181:264–272
Blaustein L (1997) Non-consumptive effects of larval Salamandra on crustacean prey: can eggs detect predators? Oecologia 110:212–217
Brown RM, Iskandar DT (2000) Nest site selection, larval hatching, and advertisement calls of Rana arathooni from southwestern Sulawesi (Celebes) Island, Indonesia. J Herpetol 34:404–413
Brown JL, Twomey E, Morales V, Summers K (2008) Phytotelm size in relation to parental care and mating strategies in two species of Peruvian poison frogs. Behaviour 145:1139–1165
Brown JL, Morales V, Summers K (2010) A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am Nat 175 (4):436–446
Buckley CR, Michael SF, Irschick DJ (2005) Early hatching decreases jumping performance in a direct-developing frog, Eleutherodactylus coqui. Funct Ecol 19:67–72
Caldwell MS, McDaniel JG, Warkentin KM (2010) Is it safe? Red-eyed treefrog embryos assessing predation risk use two features of rain vibrations to avoid false alarms. Anim Behav 79:255–260
Capellán E, Nicieza AG (2007) Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol Ecol 21:445–458
Capellán E, Nicieza A (2010) Constrained plasticity in switching across life stages: pre- and post-switch predators elicit early hatching. Evol Ecol 24:49–57
Chivers DP, Kiesecker JM, Marco A, DeVito J, Anderson MT, Blaustein AR (2001) Predator-induced life history changes in amphibians: egg predation induces hatching. Oikos 92:135–142
Delia JRJ, Ramírez-Bautista A, Summers K (2014) Glassfrog embryos hatch early after parental desertion. Proc R Soc B 281:20133237
Doody JS (2011) Environmentally cued hatching in reptiles. Integr Comp Biol 51(1):49–61
Doody JS, Stewart B, Camacho C, Christian K (2012) Good vibrations? Sibling embryos expedite hatching in a turtle. Anim Behav 83:645–651
Ferrari MCO, Chivers DP (2010) The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav Ecol Sociobiol 64:549–555
Gibbons ME, George MP (2013) Clutch identity and predator-induced hatching affect behavior and development in a leaf-breeding treefrog. Oecologia 171:831–843
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Ireland DH, Wirsing AJ, Murray DL (2007) Phenotypically plastic responses of green frog embryos to conflicting predation risk. Oecologia 152:162–168
Ishimatsu A, Graham JB (2011) Roles of environmental cues for embryonic incubation and hatching in mudskippers. Integr Comp Biol 51(1):38–48
IUCN Red List of Threatened Species. Version 2013.2 (2013) www.iucnredlist.org. Accessed 12 Feb 2014
Johnson JB, Saenz D, Adams CK, Conner RN (2003) The influence of predator threat on the timing of a life-history switch point: predator-induced hatching in the southern leopard frog (Rana sphenocephala). Can J Zool 81:1608–1613
Jozet-Alves C, Hebert M (2013) Embryonic exposure to predator odour modulates visual lateralization in cuttlefish. Proc R Soc Lond B 280:20122575
Lehman EM, Campbell CD (2007) Developmental window of response to predator chemical cues in rough-skinned newt embryos. Funct Ecol 21:880–885
Li D (2002) Hatching responses of subsocial spitting spiders to predation risk. Proc R Soc Lond B 269:2155–2161
Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP (2008) Learning by embryos and the ghost of predation future. Proc R Soc B 275:2603–2607
Miner BG, Donovan DA, Andrews KE (2010) Should I stay or should I go: predator- and conspecific-induced hatching in a marine snail. Oecologia 163:69–78
Nelson AB, Alemadi SD, Wisenden BD (2013) Learned recognition of novel predator odour by convict cichlid embryos. Behav Ecol Sociobiol 67:1269–1273
Poo S, Bickford DP (2013) The adaptive significance of egg attendance in a South-east Asian tree frog. Ethology 119:671–679
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org
Saenz D, Johnson JB, Adams CK, Dayton GH (2003) Accelerated hatching of southern leopard frog (Rana sphenocephala) eggs in response to the presence of a crayfish (Procambarus nigrocinctus) predator. Copeia 2003:646–649
Sih A, Moore RD (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat 142:947–960
Strathmann RR, Strathmann MF, Ruiz-Jones G, Hadfield MG (2010) Effect of plasticity in hatching on duration as a precompetent swimming larva in the nudibranch Phestilla sibogae. Invertebr Biol 129:309–318
Touchon JC, Gomez-Mestre I, Warkentin KM (2006) Hatching plasticity in two temperate anurans: responses to a pathogen and predation cues. Can J Zool 84:556–563
Touchon JC, McCoy MW, Vonesh JR, Warkentin KM (2013) Effects of plastic hatching timing carry over through metamorphosis in red-eyed treefrogs. Ecology 94:850–860
Vonesh JR, Bolker BM (2005) Compensatory larval responses shift trade-offs associated with predator-induced hatching plasticity. Ecology 86:1580–1591
Warkentin KM (1995) Adaptive plasticity in hatching age—a response to predation risk trade-offs. Proc Natl Acad Sci U S A 92:3507–3510
Warkentin KM (1999) The development of behavioral defenses: a mechanistic analysis of vulnerability in red-eyed tree frog hatchlings. Behav Ecol 10:251–262
Warkentin KM (2000) Wasp predation and wasp-induced hatching of red-eyed treefrog eggs. Anim Behav 60:503–510
Warkentin KM (2005) How do embryos assess risk? Vibrational cues in predator-induced hatching of red-eyed treefrogs. Anim Behav 70:59–71
Warkentin KM (2011a) Environmentally cued hatching across taxa: embryos respond to risk and opportunity. Integr Comp Biol 51:14–25
Warkentin KM (2011b) Plasticity of hatching in amphibians: evolution, trade-offs, cues and mechanisms. Integr Comp Biol 51:111–127
Warkentin KM, Caldwell MS, McDaniel JG (2006) Temporal pattern cues in vibrational risk assessment by embryos of the red-eyed treefrog, Agalychnis callidryas. J Exp Biol 209:1376–1384
Werner EE (1986) Amphibian metamorphosis—growth-rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size structured populations. Annu Rev Ecol Syst 15:393–425
Willink B, Palmer MS, Landberg T, Vonesh JR, Warkentin KM (2013) Environmental context shapes immediate and cumulative costs of risk-induced early hatching. Evol Ecol 1–14
Acknowledgments
We thank Director T. Artchawakom and the entire staff of Sakaerat for their unwavering logistic support and hospitality. We thank K.M. Warkentin for early discussions, directions, and comments on the manuscript and L.R. Carrasco for advice on statistical analyses. We are grateful to S.D. Howard and three anonymous reviewers for their constructive suggestions. Collection of field data was made possible by the wonderful assistance of A.F. McNear, J.J. Reinig, and J.S. Sherrock. Katydid species was identified by M.K. Tan and illustrations were made by A.K.S. Wee. Funding support was provided by the Ministry of Education and the National University of Singapore (Grant # R-154-000-383-133) and the Singapore International Graduate Award.
Ethical standards
All experiments were approved by the Institutional Animal Care and Use Committee at National University of Singapore (Protocol B11/12). Frog handling was done with care and all animals were returned to their original locations as soon as the experiments concluded. In addition, work at the Sakaerat Environmental Research Station was approved by the National Research Council of Thailand (Project I.D.: 2010/063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Gibbons
Rights and permissions
About this article
Cite this article
Poo, S., Bickford, D.P. Hatching plasticity in a Southeast Asian tree frog. Behav Ecol Sociobiol 68, 1733–1740 (2014). https://doi.org/10.1007/s00265-014-1781-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1781-0