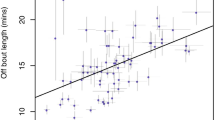
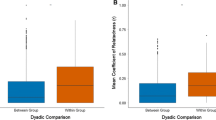
Abstract
Males of many species theoretically face a fitness tradeoff between mating and parental effort, but quantification of this is rare. We estimated the magnitude of the mating opportunity cost paid by incubating male Temminck’s stints (Calidris temminckii), taking advantage of uniparental care provided by both sexes in this species. “Incubating males” provide all care for an early clutch, limiting subsequent mating possibilities. “Non-incubating” males include males that failed to obtain, lost to predation, or actively avoided incubating clutches. These males were free to pursue extrapair copulations and to mate with females laying later clutches, which females usually incubate themselves. Male incubation classes did not differ in measures of quality, and many individuals changed classes between years, suggesting the use of conditional reproductive tactics. However, specialist non-incubators may also exist. Using microsatellites to assign parentage, we compare males’ total fertilizations and the subset “free of care” fertilizations between incubation classes. Incubators were more likely to gain at least one fertilization per season and averaged one more per season than non-incubators. However, successful non-incubators were more likely to gain “free of care” fertilizations, averaging two more than successful incubators. The relative success of male incubation classes also changed with local sex ratios. With higher female proportions, non-incubators gained disproportionately more offspring, suggesting that the use of tactics should be partly determined by the availability of potentially incubating females. Overall, we estimate the opportunity cost of incubating to be 13–25 % of the potential annual reproductive output.


Similar content being viewed by others
References
Alonso SH (2009) Social and evolutionary feedbacks between mating and parental investment. Trends Ecol Evol 25:99–107
Breiehagen T (1989) Nesting biology and mating system in an alpine population of Temminck’s stint Calidris temminckii. Ibis 131:389–402
Donald PJ (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Donald PJ (2011) Lonely males and low lifetime productivity in small populations. Ibis 153:465–467
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Emlen ST, Wrege PH, Webster MS (1998) Cuckoldry as a cost of polyandry in the sex-role-reversed wattled jacana, Jacana jacana. Proc R Soc Lond B 265:2359–2364
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Griffiths R, Double MC, Orr K, Dawson JG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–97
Harrison F, Barta Z, Cuthill IC, Székely T (2009) How is sexual conflict over parental care resolved? A meta-analysis. J Evol Biol 22:1800–1812
Hildén O (1965) Zur brutbiologie des Temminckstrandläufers, Calidris temminckii (Leisl.). Ornis Fenn 49:1–5, In German
Hildén O (1975) Breeding system of Temminck’s stint Calidris temminckii. Ornis Fenn 52:117–146
Hildén O (1979) Territoriality and site tenacity of Temminck’s stint Calidris temminckii. Ornis Fenn 56:56–74
Johnsen A, Lifjeld JT, Rohde PA, Primmer CR, Ellegren H (1998) Sexual conflict over fertilizations: female bluethroats escape male paternity guards. Behav Ecol Sociobiol 43:401–408
Jukema J, Piersma T (2006) Permanent female mimics in a lekking shorebird. Biol Lett 2:161–164
Koivula K, Rönkä A (1998) Habitat deterioration and efficiency of antipredator strategy in a meadow-breeding wader, Temminck’s stint (Calidris temminckii). Oecologia 116:348–355
Koivula K, Pakanen V-M, Rönkä A, Belda E-J (2008) Steep past and future population decline in an arctic wader: dynamics and viability of Baltic Temminck’s stints Calidris temminckii. J Avian Biol 39:329–340
Kokhanov VD (1973) Materials on the biology of the Temminck’s stint in Kandalaksha Bay in the White Sea. In: Flint VE (ed) Fauna and ecology of waders, vol 1. Moscow University Press, Moscow, pp 66–71
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Krüger O (2008) Alternative reproductive tactics in birds. In: Oliveira RF, Taborsky M, Brockmann HJ (eds) Alternative reproductive tactics: an integrative approach. Cambridge University Press, Cambridge, pp 343–355
Lank DL, Smith CM, Hanotte O, Burke TA, Cooke F (1995) Genetic polymorphism for alternative mating strategies in lekking male ruff, Philomachus pugnax. Nature 378:59–62
Lazarus J, Inglis IR (1986) Shared and unshared parental investment, parent–offspring conflict and brood size. Anim Behav 34:1791–1804
Lessells CM (2006) The evolutionary outcome of sexual selection. Philos Trans R Soc B 361:301–317
Liebezeit JR, Smith PA, Lanctot RB, Schekkerman H, Tulp I et al (2007) Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109:32–47
Liker A, Freckleton RP, Székely T (2013) The evolution of sex roles in birds is related to adult sex ratio. Nat Commun 4:1587
Low BS (1978) Environmental uncertainty and the parental strategies of marsupials and placentals. Am Nat 112:197–213
Magrath MJL, Elgar MA (1997) Paternal care declines with increasing opportunity for extra pair matings in fairy martins. Proc R Soc Lond B 264:1731–1736
Magrath MJL, Komdeur J (2003) Is male care compromised by additional mating opportunity. Trends Ecol Evol 18:424–430
Marshall T, Slate J, Kruuk L, Pemberton J (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
McNamara JM, Székely T, Webb JN, Houston AI (2000) A dynamic game theoretic model of parental care. J Theor Biol 205:605–623
Møller AP, Birkhead TR (1993) Certainty of paternity covaries with parental care in birds. Behav Ecol Sociobiol 33:261–268
Mulard H, Danchin E, Talbot SL, Ramey AM, Hatch SA, White JF, Helfenstein F, Wagner RH (2009) Evidence that pairing with genetically similar mates is maladaptive in a monogamous bird. BMC Evol Biol 9:147
Olson VA, Webb TJ, Freckleton RP, Székely T (2009) Are parental care trade-offs in shorebirds driven by parental investment or sexual selection. J Evol Biol 22:672–682
Oring LW, Fleischer RC, Reed JM, Marsden KE (1992) Cuckoldry through stored sperm in the sequentially polyandrous spotted sandpiper. Nature 359:631–633
Pakanen V-M (2011) Linking demography with dispersal and habitat selection for species conservation. Ph.D. dissertation, Acta Universitatis Ouluensis A583
Pakanen V-M, Rönkä A, Belda E-J, Luukkonen A, Kvist L, Koivula K (2010) Impact of dispersal status on estimates of local population growth rates in a Temminck’s stint (Calidris temminckii) population. Oikos 119:1493–1503
Pakanen V-M, Hildén O, Rönkä A, Belda E-J, Luukkonen A, Kvist L, Koivula K (2011) Breeding dispersal strategies following reproductive failure explain low apparent survival of immigrant Temminck’s stints. Oikos 120:615–622
Parker GA (2006) Sexual conflict over mating and fertilization: an overview. Philos Trans R Soc B 361:235–259
Pauliny A, Larsson M, Blomqvist D (2008) Nest predation management: effects on reproductive success in endangered shorebirds. J Wildl Manag 72:1579–1583
Pienkowski MW, Greenwood JJD (1979) Why change mates? Biol J Linn Soc 12:85–94
Pierce EP, Oring LW, Røskaft E, Lifjeld JT (2010) Why don’t female purple sandpipers perform brood care? A removal experiment. Behav Ecol 21:275–283
Pitcher TE, Stutchbury BJM (2000) Extraterritorial forays and male parental care in hooded warblers. Anim Behav 59:1261–1269
Pitelka FA, Holmes RT, MacLean SF Jr (1974) Ecology and evolution of social organization in Arctic sandpipers. Am Zool 14:185–204
Pogány A, Szentirmai I, Komdeur J, Székely T (2008) Sexual conflict and consistency of offspring desertion in Eurasian penduline tit Remiz pendulinus. BMC Evol Ecol 8:242
Primmer CR, Møller AP, Ellegren H (1995) Resolving genetic-relationships with microsatellite markers: a parentage testing system for the swallow Hirundo rustica. Mol Ecol 4:493–498
Qvarnström A (1997) Experimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc R Soc Lond B 264:1225–1231
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rönkä A (1996) Distribution, status and population trends in the Temminck’s stint Calidris temminckii in the Finnish Bothnian Bay. Ornis Fenn 73:1–11
Rönkä A, Koivula K, Ojanen M, Pakanen V-M, Pohjoismäki M, Rannikko K, Rauhala P (2006) Increased nest predation in a declining and threatened Temminck’s stint Calidris temminckii population. Ibis 148:55–65
Rönkä A, Kvist L, Karvonen J, Koivula K, Pakanen V-M, Schamel D, Tracy DM (2008) Population genetic structure in the Temminck’s stint Calidris temminckii, with an emphasis on Fennoscandian populations. Conserv Genet 9:29–37
Saether SA, Fiske P, Kalas JA, Kuresoo A, Luigujoe L, Piertney SB, Sahlman T, Hoglund J (2007) Inferring local adaptation from Q(ST)-F-ST comparison: neutral genetic and quantitative trait variation in European populations of great snipe. J Evol Biol 20:1563–1576
Schamel D, Tracy DM, Lank DB, Westneat DF (2004) Mate guarding, copulation strategies and paternity in the sex-role reversed, socially polyandrous red-necked phalarope Phalaropus lobatus. Behav Ecol Sociobiol 57:110–118
Schuster SM, Wade MJ (2003) Mating systems and sexual strategies. Princeton University Press, Princeton
Smith HG (1995) Experimental demonstration of a trade-off between mate attraction and parental care. Proc R Soc Lond B 260:45–51
Smith PA, Wilson S (2010) Intraseasonal patterns in shorebird nest survival are related to nest age and defence behaviour. Oecologia 163:613–624
Smith PA, Gilchrist HG, Smith JNM (2007) Effects of nest habitat, food, and parental behavior on shorebird nest success. Condor 109:15–31
Stiver KA, Alonso SH (2009) Parental and mating effort: is there necessarily a trade-off. Ethology 115:1101–1126
Sundström T, Olsson C (2009) Norrbottens kustfågelbestånd–inventering 2007 och 2008. Länstyrelsens rapportserie nr 5/2009. Länsstyrelsen, Luleå, In Swedish
Székely T, Cuthill IC (2000) Trade-off between mating opportunities and parental care: brood desertion by female Kentish plovers. Proc R Soc Lond B 267:2087–2092
Székely T, Cuthill IC, Kis J (1999) Brood desertion in Kentish plover: sex differences in remating opportunities. Behav Ecol 10:185–190
Székely T, Kosztolányi A, Küpper C, Thomas GH (2007) Sexual conflict over parental care: a case study of shorebirds. J Ornithol 148:S211–S217
Szentirmai I, Székely T, Komdeur J (2007) Sexual conflict over care: antagonistic effects of clutch desertion on reproductive success of male and female penduline tits. J Evol Biol 20:1739–1744
Thomas GH, Székely T, Reynolds JD (2007) Sexual conflict and the evolution of breeding systems in shorebirds. Adv Study Behav 37:279–342
Thuman KA, Widemo F, Piertney SB (2002) Characterization of polymorphic microsatellite DNA markers in the ruff (Philomachus pugnax). Mol Ecol Notes 2:276–277
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago, pp 136–179
van Treuren R, Bijlsma R, Tinbergen JM, Heg D, Zande L (1999) Genetic analysis of the population structure of socially organized oystercatchers (Haematopus ostralegus) using microsatellites. Mol Ecol 8:181–187
von Oosterhout C, Hutchinson WF, Wills PM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Wennerberg L, Bensch S (2001) Geographic variation in the dunlin Calidris alpina as revealed by morphology, mtDNA and microsatellites. In: Wennerberg L (ed) Genetic variation and migration in waders [dissertation]. Lund University, Sweden, pp 43–55
Westerskov K (1950) Methods for determining the age of game bird eggs. J Wildl Manag 14:56–67
Westneat DF (1988) Male parental care and extrapair copulations in the indigo bunting. Auk 105:149–160
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: causes, correlates, and conflict. Annu Rev Ecol Evol Syst 34:365–396
Whitfield DP (1990) Male choice and sperm competition as constraints on polyandry in the red-necked phalarope Phalaropus lobatus. Behav Ecol Sociobiol 27:247–254
Whittingham LA, Dunn PO (2001) Male parental care and paternity in birds. Curr Ornithol 16:257–298
Williams GC (1966) Adaptation and natural selection. Princeton University Press, Princeton
Wisenden BD (1999) Alloparental care in fishes. Rev Fish Biol Fish 9:45–70
Acknowledgments
We thank Aappo Luukkonen and Riku Halmeenpää for help in the field. DMT thanks Sandy Talbot, Kevin Sage, and Judy Gust for their patience and help in the US Geological Survey, Alaska Science Center lab. David Westneat and two anonymous reviewers provided constructive feedback on earlier versions of this manuscript. This study was supported by the USGS Alaska Science Center, the National Science and Engineering Council of Canada (to DBL), the Finnish Cultural Foundation, Kone Foundation, Emil Aaltonen Foundation (to VMP), the Finnish Environment Centre, and the Academy of Finland (project 128384 to KK; project 138049 to RLT).
Ethical standards
This study complied with the current laws of Finland. The ringing of birds was done under permit from the Finnish Museum of Natural History. Blood sampling and disturbance of a breeding birds was approved by the Centre for Economic Development, Transport and the Environment in North Ostrobothnia, Finland (Dnro 1101L0353-254 and PPO-2006-L-206-254).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. R. Brown
Robert L. Thomson and Veli-Matti Pakanen joint first authors with equal contributions to this manuscript.
Rights and permissions
About this article
Cite this article
Thomson, R.L., Pakanen, VM., Tracy, D.M. et al. Providing parental care entails variable mating opportunity costs for male Temminck’s stints. Behav Ecol Sociobiol 68, 1261–1272 (2014). https://doi.org/10.1007/s00265-014-1737-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1737-4