Abstract
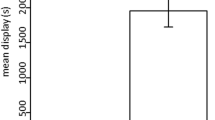
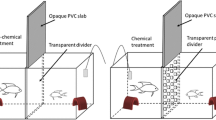
Communication plays a large role in resource competition, especially for potential mates, and is used by members of the competing sex to assess each other, and simultaneously to evaluate the other sex, which may be advertising its status. To assess the effects of female advertisement on male aggression, males of the decapod Aegla were paired according to body and armament size. Males were left to interact in five different treatments: with receptive females that could use both chemical and visual cues, non-receptive females that could use both types of cues, receptive females that could use only one cue, or no female in the aquarium. Fight duration, time spent in the most aggressive acts, latency period, number of antennal whips/fight duration, and time spent near the female were analyzed. The males had shorter and less intense confrontations when there was a receptive female that could signal with at least one modality. Winning males spent significantly more time near the receptive female only when both chemical and visual cues were present, when compared to the other treatments. The low level of aggression shown by the males may be related to information asymmetry due to the female’s choice: only the preferred male would receive information from the female, or males could compete for other resources that attract females. However, male aggression was modified by the presence of female chemical cues, whereas mate guarding was initiated only when both chemical and visual cues were present. Hence, male aggression can be downregulated by female receptivity.





Similar content being viewed by others
References
Acquistapace P, Aquiloni L, Hazlett BA, Gherardi F (2002) Multimodal communication in crayfish: sex recognition during mate search by male Austropotamobius pallipes. Can J Zool 80:2041–2045
Almerão M, Bond-Buckup G, Mendonça MS Jr (2010) Mating behavior of Aegla platensis (Crustacea, Anomura, Aeglidae) under laboratory conditions. J Ethol 28:87–94
Aquiloni L, Gherardi F (2008) Assessing mate size in the red swamp crayfish Procambarus clarkii: effects of visual versus chemical stimuli. Freshwater Biol 53:461–469
Aquiloni L, Gherardi F (2010) Crayfish females eavesdrop on fighting males and use smell and sight to recognize the identity of the winner. Anim Behav 79:265–269
Aquiloni L, Gonçalves V, Inghilesi AF, Gherardi F (2012) Who’s what? Prompt recognition of social status in crayfish. Behav Ecol Sociobiol 66:785–790
Asakura A (2009) The evolution of mating systems in decapod crustaceans. In: Martin JW, Crandal KA, Felder DL (eds) Decapod crustacean phylogenetics. CRC Press, Boca Raton, pp 121–183
Atema J (1995) Chemical signals in the marine environment: dispersal, detection, and temporal signal analysis. Proc Natl Acad Sci U S A 92:62–66
Atema J, Cowan DF (1986) Sex-identifying urine and molt signals in the lobster Homarus americanus. J Chem Ecol 12:2065–2080
Atema J, Steinbach MA (2007) Chemical communication in the lobster, Homarus americanus, and other decapod Crustacea. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems—crustaceans as model organisms. Oxford University Press, New York, pp 115–147
Ayres-Peres L, Araujo PB, Santos S (2011a) Description of the agonistic behavior of Aegla longirostri (Decapoda: Aeglidae). J Crustacean Biol 31(3):379–388
Ayres-Peres L, Coutinho C, Baumart JS, Gonçalves AS, Araujo PB, Santos S (2011b) Radio-telemetry techniques in the study of displacement of freshwater anomurans. Nauplius 19(1):41–54
Bergman DA, Moore PA (2003) Field observations of intraespecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol Bull 205:26–35
Bergman DA, Moore PA (2005) Prolonged exposure to social odours alters subsequent social interactions in crayfish (Orconectes rusticus). Anim Behav 70:311–318
Berry FC, Breithaupt T (2008) Development of behavioural and physiological assays to assess discrimination of male and female odours in crayfish, Pacifastacus leniusculus. Behaviour 145:1427–1446
Berry FC, Breithaupt T (2010) To signal or not to signal? Chemical communication by urine-borne signals mirrors sexual conflict in crayfish. BMC Biol 8:25
Breithaupt T (2011) Chemical communication in crayfish. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 257–276
Breithaupt T, Atema J (2000) The timing of chemical signaling with urine in dominance fights of male lobsters (Homarus americanus). Behav Ecol Sociobiol 49:67–78
Breithaupt T, Eger P (2002) Urine makes the difference: chemical communication in fighting crayfish made visible. J Exp Biol 205:1221–1231
Bro-Jørgensen J (2007) The intensity of sexual selection predicts weapon size in male bovids. Evolution 61:1316–1326
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Bücker F, Gonçalves R, Bond-Buckup G, Melo AS (2008) Effect of environmental variables on the distribution of two freshwater crabs (Anomura: Aeglidae). J Crustacean Biol 28(2):248–251
Bueno AAP, Bond-Buckup G (2000) Dinâmica populacional de Aegla platensis (Crustacea, Decapoda, Aeglidae). Rev Bras Zool 17(1):43–49
Bueno SLS, Shimizu RM, Rocha SS (2007) Estimating the population size of Aegla franca (Decapoda: Anomura: Aeglidae) by mark-recapture technique from an isolated section of Barro Preto Stream, county of Claraval, State of Minas Gerais, Southeastern Brazil. J Crustacean Biol 27(4):553–559
Bushmann PJ, Atema J (2000) Chemically mediated mate location and evaluation in the lobster, Homarus americanus. J Chem Ecol 26:883–899
Christy JH, Rittschof D (2011) Deception in visual and chemical communication in crustaceans. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 313–333
Colpo KD, Oliveira LR, Santos S (2005) Population biology of the freshwater anomuran Aegla longirostri (Crustacea, Anomura, Aeglidae) from Ibicuí-Mirim River, Itaára, RS, Brazil. J Crustacean Biol 25:495–499
Dalosto M, Santos S (2011) Differences in oxygen consumption and diel activity as adaptations related to microhabitat in Neotropical freshwater decapods (Crustacea). Comp Biochem Phys A 160:461–466
Dennenmoser S, Thiel M (2007) Competition for food and mates by dominant and subordinate male rock shrimp, Rhynchocinetes typus. Behaviour 144:33–59
Díaz ER, Thiel M (2004) Chemical and visual communication during mate searching in rock shrimp. Biol Bull 206:134–143
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Enquist M, Leimar O (1987) Evolution of fighting behavior: the effect of variation in resource value. J Theor Biol 127:187–205
Fletcher N, Hardege JD (2009) The cost of conflict: agonistic encounters influence responses to chemical signals in the European shore crab. Anim Behav 77:357–361
Gherardi F (2002) Behaviour. In: Holdich DM (ed) Biology of freshwater crayfish. Blackwell Science, Oxford, pp 258–290
Gherardi F, Atema J (2005) Memory of social partners in hermit crab dominance. Ethology 111:271–285
Gherardi F, Tricarico E (2011) Chemical ecology and social behavior of Anomura. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 297–313
Goessmann C, Hemelrijk C, Huber R (2000) The formation and maintenance of crayfish hierarchies: behavioral and self-structuring properties. Behav Ecol Sociobiol 48:418–428
Goldberg JL, Grant JWA, Lefebvre L (2001) Effects of the temporal predictability and spatial clumping of food on the intensity of competitive aggression in the Zenaida dove. Behav Ecol 12:490–495
Grant JWA, Gaboury CL, Levitt HL (2000) Competitor-to-resource ratio, a general formulation of operational sex ratio, as a predictor of competitive aggression in Japanese medaka (Pisces: Oryziidae). Behav Ecol 11:670–675
Guilford T, Dawkins MS (1991) Receiver psychology and the evolution of animal signals. Anim Behav 42:1–14
Hebets EA, Rundus AS (2011) Chemical communication in a multimodal context. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 335–354
Hofmann HA, Schildberger K (2001) Assessment of strength and willingness to fight during aggressive encounters in crickets. Anim Behav 62:337–348
Hölldobler B (1999) Multimodal signals in ant communication. J Comp Physiol A 184:129–141
Hughes M (1996) The function of concurrent signals: visual and chemical communication in snapping shrimp. Anim Behav 52:247–257
Hunt J, Breuker CJ, Sadowski JA, Moore AJ (2009) Male–male competition, female mate choice and their interaction: determining total sexual selection. J Evolution Biol 22:13–26
Jivoff P, Hines AH (1998) Effect of female molt stage and sex ratio on courtship behavior of the blue crab Callinectes sapidus. Mar Biol 131:533–542
Kamio M, Reidenbach MA, Derby CD (2008) To paddle or not: context dependent courtship display by male blue crabs, Callinectes sapidus. J Exp Biol 211:1243–1248
Karavanich C, Atema J (1998) Olfactory recognition of urine signals in dominance fights between male lobster, Homarus americanus. Behaviour 135(6):719–730
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evolution Biol 21:919–948
Lott DF (1991) Intraspecific variation in the social systems of wild vertebrates. Cambridge University Press, Cambridge
Maynard Smith J (1974) The theory of games and the evolution of animal conflicts. J Theor Biol 47:209–221
Moore PA (2007) Agonistic behaviour in freshwater crayfish: the influence of intrinsic and extrinsic factors on aggressive encounters and dominance. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems—Crustaceans as model organisms. Oxford University Press, New York, pp 91–112
Munoz NE, Blumstein DT (2012) Multisensory perception in uncertain environments. Behav Ecol 23:457–462
Nonacs P, Blumstein DT (2010) Predation risk and behavioral life history. In: Westneat DF, Fox CW (eds) Evolutionary behavioral ecology. Oxford University Press, New York, pp 207–221
Parker GA (1974) Assessment strategy and the evolution of fighting behavior. J Theor Biol 47:223–242
Partan S, Marler P (1999) Communication goes multimodal. Science 283:1272–1273
Partan S, Marler P (2005) Issues in the classification of multimodal communication signals. Am Nat 166:231–245
Pérez-Barros P, Calcagno JA, Lovrich GA (2011) Absence of a prezygotic behavioural barrier to gene flow between the two sympatric morphs of the squat lobster Munida gregaria (Fabricius, 1793) (Decapoda: Anomura: Galatheidae). Helgol Mar Res 65:513–523
Development Core Team R (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rohr JR, Park D, Sullivan AM, McKenna M, Propper CR, Madison DM (2004) Operational sex ratio in newts: field responses and characterization of a constituent chemical cue. Behav Ecol 16:286–293
Rowe C, Guilford T (1999) The evolution of multimodal warning displays. Evol Ecol 13:655–671
Salmon M (1983) Courtship, mating systems; and sexual selection in decapods. In: Rebach S, Dunham D (eds) Studies in adaptation: the behavior of higher crustaceans. Wiley, New York, pp 143–169
Santos S, Ayres-Peres L, Cardoso RCF, Sokolowicz CC (2007) Natural diet of the freshwater anomuran Aegla longirostri (Crustacea, Anomura, Aeglidae). J Nat Hist 42:1027–1037
Schütz D, Taborsky M (2011) Sexual selection in the water spider: female choice and male–male competition. Ethology 117:1101–1110
Smallegange IM, Sabelis MW, van der Meer J (2007) Assessment games in shore crab fights. J Exp Mar Biol Ecol 351:255–266
Skog M, Chandrapavan A, Hallberg E, Breithaupt T (2009) Maintenance of dominance is mediated by urinary chemical signals in male European lobsters, Homarus gammarus. Mar Freshw Behav Phy 42(2):119–133
Sneddon LU, Huntingford FA, Taylor AC, Clare AS (2003) Female sex pheromone-mediated effects on behavior and consequence of male competition in the shore crab (Carcinus maenas). J Chem Ecol 29(1):55–70
Sokolowicz CC, López-Greco LS, Gonçalves R, Bond-Buckup G (2007a) The gonads of Aegla platensis Schmitt (Decapoda, Anomura, Aeglidae): a macroscopic and histological perspective. Acta Zool-Stockholm 88:71–79
Sokolowicz CC, Ayres-Peres L, Santos S (2007b) Atividade nictimeral e tempo de digestão de Aegla longirostri (Crustacea, Decapoda, Anomura). Iheringia Sér Zool 97(3):235–238
Takács S, Mistal C, Gries G (2003) Communication ecology of webbing clothes moth: attractiveness and characterization of male-produced sonic aggregation signals. J Appl Entomol 127:127–133
Thiel M, Breithaupt T (2011) Chemical communication in crustaceans: research challenges for the twenty-first century. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 3–23
Thiel M, Correa C (2004) Female rock shrimp Rhynchocinetes typus mate in rapid succession up a male dominance hierarchy. Behav Ecol Sociobiol 57:62–68
Thiel M, Espinoza-Fuenzalida NL, Acuña E, Rivadeneira MM (2012) Annual brood number and breeding productivity of squat lobsters (Decapoda: Anomura: Galatheidae) from the continental shelf of the SE Pacific—implications for fisheries management. Fish Res 123–129:28–37
Thiel M, Hinojosa I (2003) Mating behavior of female rock shrimp Rhynchocinetes typus (Decapoda: Caridea) indication for convenience polyandry and cryptic female choice. Behav Ecol Sociobiol 55:113–121
Turra A, Denadai MR (2003) Daily activity of four tropical intertidal hermit crabs from southeastern Brazil. Braz J Biol 63:537–544
Vahl WK, Lok T, van der Meer J, Piersma T, Weissing FJ (2005) Spatial clumping of food and social dominance affect interference among ruddy turnstones. Behav Ecol 16:834–844
Van der Velden J, Zheng Y, Patullo BW, Macmillan DL (2008) Crayfish recognize the faces of fight opponents. PLoS One 3(2):e1695. doi:10.1371/journal.pone.0001695
Viau VE, Greco LSL, Bond-Buckup G, Rodríguez EM (2006) Size at the onset of sexual maturity in the anomuran crab, Aegla uruguayana (Aeglidae). Acta Zool-Stockholm 87:253–264
Weissburg MJ (2011) Waterborne chemical communication: stimulus dispersal dynamics and orientation strategies in crustaceans. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 63–85
Wyatt TD (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for individuality in both invertebrates and vertebrates. J Comp Physiol A 196:685–700
Zimmermann BL, Aued AW, Machado S, Manfio D, Scarton LP, Santos S (2009) Behavioral repertory of Trichodactylus panoplus (Crustacea: Trichodactylidae) under laboratory conditions. Zoologia 26(1):5–11
Acknowledgments
The authors are grateful to MSc. Marcelo Marchet Dalosto and Aimée Ferreira Siqueira for their help with the experiments and to MSc. Francisco Diogo Rocha Sousa for his help with the figures. They are also grateful to the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the scholarship to AVP, the doctoral scholarship to LAP, and the productivity grant to SS; to an anonymous reviewer for his comments of the manuscript; and to Dr. Martin Thiel for his extensive comments, which helped to substantially improve the manuscript, and his help through the entire editorial process.
Ethical standards
All animals were sampled, maintained, and returned to the natural environment under license from IBAMA (Instituto Brasileiro do Meio Ambiente), number 14180–1, granted on December 4, 2007 and according to the applicable statutes (Law number 5197, of January 3, 1967; Resolutions: number 16 of March 4, 1994 and number 332 of March 13, 1990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Breithaupt
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 38 kb)
Rights and permissions
About this article
Cite this article
Palaoro, A.V., Ayres-Peres, L. & Santos, S. Modulation of male aggressiveness through different communication pathways. Behav Ecol Sociobiol 67, 283–292 (2013). https://doi.org/10.1007/s00265-012-1448-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1448-7