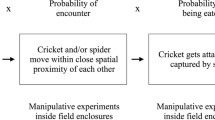
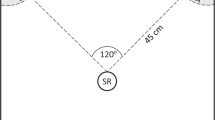
Abstract
Theories of lek evolution generally invoke enhanced mating success experienced by males signalling in aggregations. Reduced predation has also been acknowledged as a potential factor driving lek formation, but its role is more ambiguous. Although lekking is a complex behaviour, few empirical studies have investigated the role of both claims. We studied the potential pressures imposed by mating success and predation in an acoustic moth, Achroia grisella, in which males gather in leks and broadcast a calling song attractive to females. We exploited the ability to manipulate the distribution of singing males in laboratory arenas to create different-sized leks and tested female preferences for these aggregations. Because A. grisella are vulnerable to predation by bats while in flight and on the substrate, we also tested the responses of a potential predator, Rhinolophus ferrumequinum, a bat species that feeds on moths, to the experimental leks. We found that the per capita attractiveness of A. grisella males to females rose with increasing lek size. R. ferrumequinum also oriented toward experimental A. grisella leks, but this attraction did not increase at larger leks. Thus, a male’s per capita exposure to predation risk declined as more moths joined the lek. A. grisella males appear to benefit from advertising in larger leks in terms of both increased mate attraction and reduced predation risk. Our results support the idea that multiple factors operating simultaneously may maintain lekking behaviour.





Similar content being viewed by others
References
Acharya L, McNeil JN (1998) Predation risk and mating behavior: the responses of moths to bat-like ultrasound. Behav Ecol 9:552–558
Alatalo RV, Höglund J, Lundberg A (1991) Lekking in black grouse—a test of male viability. Nature 352:155–156
Alem S, Greenfield MD (2010) Economics of mate choice at leks: do female waxmoths pay costs for indirect genetic benefits? Behav Ecol 21:615–625
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arlettaz R, Jones G, Racey PA (2001) Effect of acoustic clutter on prey detection by bats. Nature 414:742–745
Balmford A (1990) Lekking in Uganda kob. Ph.D. thesis, Cambridge University
Balmford A, Turyaho M (1992) Predation risk and lek breeding in Uganda kob. Anim Behav 44:117–127
Beehler BM (1988) Lek behaviour of the Raggiana bird of paradise. Natn Geogr Res 4:343–358
Beehler BM, Foster MS (1988) Hotshots, hotspots and female preference in the organization of lek mating systems. Am Nat 131:203–219
Booth-Binczik SD, Binczik GA, Labisky RF (2004) Lek-like mating in white-nosed coatis (Nasua narica): socio-ecological correlates of intraspecific variability in mating systems. J Zool Lond 262:179–185
Boyko AR, Gibson RM, Lucas JR (2004) How predation risk affects the temporal dynamics of avian leks: Greater sage grouse versus golden eagles. Am Nat 163:154–165
Bradbury JW, Gibson RM (1983) Leks and mate choice. In: Bateson P (ed) mate choice. Cambridge University Press, Cambridge, pp 109–138
Bradbury JW, Gibson RM, McCarthy CE, Vehrencamp SL (1989) Dispersion of displaying male sage grouse II: the role of female dispersion. Behav Ecol Sociobiol 24:15–24
Brandt LSE (2003) Evolutionary origin and consequences of female mate choice in an ultrasonic moth, Achroia grisella. Dissertation, University of Kansas
Brunel-Pons O, Alem S, Greenfield MD (2011) The complex auditory scene at leks: balancing antipredator and competitive signalling in an acoustic moth. Anim Behav 81:231–239
Brunner E, Domhorf S, Langer F (2002) Nonparametric analysis of longitudinal data in factorial experiments. Wiley, New York
Cade WH (1981) Alternative mating strategies: genetic differences in crickets. Science 212:563–564
Candolin U (1997) Predation risk affects courtship and attractiveness of competing threespine stickleback males. Behav Ecol Sociobiol 41:81–87
Conner WE (1999) ‘Un chant d’appel amoureux’: acoustic communication in moths. J Exp Biol 202:1711–1723
Corcoran AJ, Barber JR, Conner WE (2009) Tiger moth jams bat sonar. Science 325:325–327
Crocker MJ (1998) Handbook of acoustics. Wiley Interscience, New York
Csorba G, Ujhelyi P, Thomas N (2003) Horseshoe bats of the world (Chiroptera: Rhinolophidae). Alana Books, Shropshire
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Deutsch JC (1994) Uganda kob mating success does not increase on larger leks. Behav Ecol Sociobiol 34:451–459
Dietz C, Nill D, von Helversen O (2007) Handbuch der Fledermäuse Europas und Nordwestafrikas. Kosmos, Stuttgart
Faure PA, Barclay RMR (1992) The sensory basis of prey detection by the long-eared bat, Myotis evotis, and the consequences for prey selection. Anim Behav 44:31–39
Gibson RM, Bachman GC (1992) The costs of female choice in a lekking bird. Behav Ecol 3:300–309
Gibson RM, Aspbury AS, McDaniel L (2002) Active formation of mixed-species grouse leks: a role for predation in lek evolution? Proc R Soc Lond B 269:2503–2508
Goerlitz HR, Greif S, Siemers BM (2008) Cues for acoustic detection of prey: insect rustlings sounds and the influence of walking substrate. J Exp Biol 211:2799–2806
Grafe TU (1997) Costs and benefits of mate choice in the lek-breeding reed frog, Hyperolius marmoratus. Anim Behav 53:1103–1117
Greenfield MD, Baker M (2003) Bat avoidance in non-aerial insects: the silence response of signaling males in an acoustic moth. Ethology 109:427–442
Greenfield MD, Coffelt JA (1983) Reproductive behaviour of the lesser wax moth, Achroia grisella (Pyralidae: Galleriinae): signalling, pair formation, male interactions, and mate guarding. Behaviour 84:287–315
Greenfield MD, Hohendorf H (2009) Independence of sexual and antipredator perceptual functions in an acoustic moth: implications for the receiver bias mechanism in signal evolution. Ethology 115:1137–1149
Greenfield MD, Weber T (2000) Evolution of ultrasonic signalling in wax moths: discrimination of ultrasonic mating calls from bat echolocation signals and the exploitation of an anti-predator receiver bias by sexual advertisement. Ethol Ecol Evol 12:259–279
Greig EI, Greenfield MD (2004) Sexual selection and predator avoidance in an acoustic moth: discriminating females take fewer risks. Behaviour 141:799–815
Griffin DR, Simmons JA (1974) Echolocation of insects by horseshoe bats. Nature 250:731–732
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton
Holm S (1979) A simple sequentially-rejective multiple test procedure. Scand J Stat 6:65–70
Isvaran K, St. Mary CM (2003) When should males lek? Insights of a dynamic state variable model. Behav Ecol 14:876–886
Jacobs DS, Ratcliffe JM, Fullard JH (2008) Beware of bats, beware of birds: the auditory responses of eared moths to bat and bird predation. Behav Ecol 19:1333–1342
Jang Y, Greenfield MD (1996) Ultrasonic communication and sexual selection in wax moths: female choice based on energy and asynchrony of male signals. Anim Behav 51:1095–1106
Jang Y, Greenfield MD (1998) Absolute versus relative measurements of sexual selection: assessing the contributions of ultrasonic signal characters to mate attraction in lesser wax moths, Achroia grisella (Lepidoptera: Pyralidae). Evolution 52:1383–1393
Jia F-Y, Greenfield MD, Collins RD (2001) Ultrasonic signal competition between male wax moths. J Insect Behav 14:19–33
Jiguet F, Bretagnolle V (2006) Manipulating lek size and composition using decoys: an experimental investigational lek evolution models. Am Nat 168:758–768
Jones G (1990) Prey selection by the greater horseshoe bat (Rhinolophus ferrumequinum): optimal foraging by echolocation? J Anim Ecol 59:587–602
Jones TM, Quinell RJ (2002) Testing predictions for the evolution of lekking in the sandfly, Lutzomyia longipalpis. Anim Behav 63:605–612
Jones G, Rayner JMV (1989) Foraging behavior and echolocation of wild horseshoe bats Rhinolophus ferrumequinum and Rhinolophus hipposideros (Chiroptera, Rhinolophidae). Behav Ecol Sociobiol 25:183–191
Jones G, Rydell J (2003) Attack and defense: interactions betweenecholocating bats and their insect prey. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, pp 301–345
Jones G, Barabas A, Elliot W, Parsons S (2002) Female greater wax moths reduce sexual display behavior in relation to the potential risk of predation by echolocating bats. Behav Ecol 13:375–380
Kalko EKV, Handley CO Jr (2001) Neotropical bats in the canopy: diversity, community structure, and implications for conservation. Plant Ecol 153:319–333
Karban R (1982) Increased reproductive success at high densities and predator satiation for periodical cicadas. Ecology 63:321–328
Kober R, Schnitzler HU (1990) Information in sonar echoes of fluttering insects available for echolocating bats. J Acoust Soc Am 87:882–896
Koivisto I (1965) Behavior of the black grouse, Lyrurus tetris (L.), during the spring display. Finn Game Res 26:5–60
Kokko H (1997) The lekking game: can female choice explain aggregated male displays? J Theor Biol 187:57–64
Koselj K, Schnitzler H-U, Siemers BM (2011) Horseshoe bats make adaptive prey-selection decisions, informed by echo cues. Proc R Soc B. doi:10.1098/rspb.2010.2793
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lafaille M, Bimbard G, Greenfield MD (2010) Risk trading in mating behavior: forgoing anti-predator responses reduces the likelihood of missing terminal mating opportunities. Behav Ecol Sociobiol 64:1485–1494
Lank DB, Smith CM (1992) Females prefer larger leks: field experiments with ruffs (Philomachus pugnax). Behav Ecol Sociobiol 30:323–329
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation—a review and prospectus. Can J Zool 68:619–640
Loiselle BA, Ryder TB, Duãres R, Tori W, Blake JG, Parkeret PG (2007) Kin selection does not explain male aggregation at leks of 4 manakin species. Behav Ecol 18:287–291
Magnhagen C (1991) Predation risk as a cost of reproduction. TREE 6:183–186
Miller LA, Surlykke A (2001) How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. Bioscience 51:570–581
Milum VG (1940) Larval pests common to the nests of bumblebees and combs of the honeybees. J Econ Entomol 33:81–83
Nakano R, Takanashi T, Fujii T, Skals N, Surlykke A, Ishikawa Y (2009) Moths are not silent, but whisper ultrasonic courtship songs. J Exp Biol 212:4072–4078
Pir JB (1994) Etho-ökologische Untersuchung einer Wochenstubenkolonie der Großen Hufeisennase (Rhinolophus ferrumequinum Schreber 1774) in Luxemburg. Diploma thesis, Justus Liebig University Giessen
Pocklington R, Dill LM (1995) Predation on females or males: who pays for bright male traits? Anim Behav 49:1122–1124
Ratcliffe JM, Nydam ML (2008) Multimodal warning signal for a multiple predator world. Nature 455:96–99
Rodriguez RL, Greenfield MD (2004) Behavioral context regulates dual function of hearing in ultrasonic moths: bat avoidance and pair formation. Physiol Entomol 29:159–168
Rodriguez RL, Cocroft RB, Schul J, Greenfield MD (2005) The contribution of tympanic transmission to fine temporal signal evaluation in an ultrasonic moth. J Exp Biol 208:4159–4165
Ryan MJ, Tuttle MD, Taft LK (1981) The costs and benefits of frog chorusing behaviour. Behav Ecol Sociobiol 8:273–278
Schnitzler HU (1987) Echoes of fluttering insects: information for echolocating bats. In: Fenton MB, Racey P, Rayner JMV (eds) Recent advances in the study of bats. Cambridge University Press, Cambridge, pp 226–243
Schnitzler HU, Kalko EKV (2001) Echlocation by insect-eating bats. Bioscience 51:557–569
Schnitzler HU, Moss CF, Denzinger A (2003) From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol 18:386–394
Schuchmann M, Siemers BM (2010) Variability in echolocation call intensity in a community of horseshoe bats: a role for resource partitioning or communication? PLoS One 5:e12842
Siemers BM, Güttinger R (2006) Prey conspicuousness can explain apparent prey selectivity. Curr Biol 16:157–159
Siemers BM, Ivanova T (2004) Ground gleaning in horseshoe bats: comparative evidence from Rhinolophus blasii, R. euryale and R. mehelyi. Behav Ecol Sociobiol 56:464–471
Spangler HG, Greenfield MD, Takessian A (1984) Ultrasonic mate calling in the lesser wax moth. Physiol Entomol 9:87–95
Stillman RA, Clutton-Brock TH, Sutherland WJ (1993) Black-holes, mate retention, and the evolution of ungulate leks. Behav Ecol 4:1–6
Trail PW (1987) Predation and antipredator behaviour at guianan cock-of-the-rock leks. Auk 104:496–507
Turchin P, Kareiva P (1989) Aggregation in Aphis varians: an effective strategy for reducing predation risk. Ecology 70:1008–1016
van Rhijn JG (1983) On the maintenance of alternative strategies in the ruff Philomachus pugnax. Ibis 125:482–498
Vogler B, Neuweiler G (1983) Echolocation in the noctule (Nyctalus noctula) and horseshoe bat (Rhinolophus ferrumequinum). J Comp Physiol A 152:421–432
von der Emde G, Schnitzler HU (1990) Classification of insects by echolocating greater horseshoe bats. J Comp Physiol A 167:423–430
Waters DA (2003) Bats and moths: what is there left to learn? Physiol Entomol 28:237–250
Weldon CW (2007) Influence of male aggregation size on female visitation in Bactrocera tryoni (Frogatt) (Diptera: Tephritidae). Austral J Entomol 46:29–34
Werner NY, Lotem A (2003) Choosy male in a haplochromine cichlid: first experimental evidence for male mate choice in a lekking species. Anim Behav 66:293–298
Westcott D, Smith J (1997) Lek size variation and its consequences in the ochre-bellied flycatcher, Mionectes oleaginous. Behav Ecol 8:396–403
Wiley RH (1991) Lekking in birds and mammals: behavioral and evolutionary issues. Adv Stud Behav 20:201–291
Young KA, Genner MJ, Joyce DA, Haesler MP (2009) Hotshots, hot spots and female preference: exploring lek formation models with a bower-building cichlid fish. Behav Ecol 20:609–615
Acknowledgements
Bats were captured and exported under permits 35701-13/2003 and 35717-20/2004 of the Environmental Agency of the Republic of Slovenia and under permit Z3.48.05/AUS 0007/03 of the German Federal Agency for Nature Conservation. Bats were housed under licence 301c.4V-sä of the Landratsamt Starnberg. We thank Pascal Binon (Tolaud, Dépt. Ardèche) for help in obtaining A. grisella; Guy Bourdais, Bruno Brizard, and Fabrice Vannier (IRBI, Tours, France) for technical assistance with moth rearing and experimentation; Renate Heckel-Merz for expert help with bat husbandry; Erich Koch for building the Seewiesen moth arena; the Agence Nationale de la Recherche de France (contrat ANR-07-BLAN-0113-01), the Centre National de la Recherche Scientifique (CNRS), the Université François Rabelais de Tours, and the Max Planck Society for their financial support. We thank Marlène Goubault, Séverine Ligout, Denis Limousin and Nathan Morehouse, and several anonymous referees for valuable criticisms of earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Jones
Rights and permissions
About this article
Cite this article
Alem, S., Koselj, K., Siemers, B.M. et al. Bat predation and the evolution of leks in acoustic moths. Behav Ecol Sociobiol 65, 2105–2116 (2011). https://doi.org/10.1007/s00265-011-1219-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1219-x